Preparation of PEG20K-Pd Nanoparticles & their Characterization
Received Date: May 11, 2024 Accepted Date: June 11, 2024 Published Date: June 14, 2024
doi: 10.17303/jnsm.2024.10.103
Citation: Shweta Gupta, Rupesh Kumar, Dhanraj T Masram (2024) Preparation of PEG20K-Pd Nanoparticles & their Characterization. J Nanotech Smart Mater 10: 1-9
Abstract
An efficient synthesis of PEG20K-Pd nanoparticles in water has been developed using PEG20K (Polyethylene ethylene glycol molecular weight 20000) as the capping agent.Prepared nanoparticles were well characterized by various techniques DLS, TEM,SEM, XRD,TGA, DSc, IR & UV. High stability of the prepared nanoparticles is due to protective polymer Polyethylene glycol molecular weight 20000 employed that fulfills the various requirements such as reducing agent and stabilizer. Stable palladium nanoparticles developed by providing low cost high molecular weight polymer polyethylene glycol 20000.This polymer is cheaply available.
Keywords: Palladium; PEG20K; Nanoparticles; Monodisperse; Water Soluble
Introduction
Nanoparticles have become attractive key materials in both academic and industrial areas because of their unique chemical and physical properties, such as catalytic, optical, and magnetic properties distinct from those of bulk metals or atoms [1-4]. Transition-metal nanoparticles have attracted a great deal of attention in the last 10 years; their preparation, structure determination, and applications are topics of current interest [5-18]. The smaller the cluster of atoms, the higher the percentage of atoms are on the surface, rendering nanoparticles very interesting in catalysis [10,15,18].Metal nanoparticles are used for catalytic hydrogenation of nitroarenes over heterogeneous catalysts. The metal atoms constituting nanoparticles can be generated by (i) chemical reduction of a metal salt, (ii) thermal, photochemical, or sonochemical decomposition of a metal(0) complex, (iii) hydrogenation of a coordinating olefinic moiety, and (iv) vapor phase deposition. To this list proposed by Bradley [6] should be added (v) electrochemical reduction of higher valent species of the metal [13(a-c)]. During generation of nanoparticles, the following steps have been identified: (i) generation of atoms as above; (ii) nucleation to form an initial cluster of atoms; (iii) growing of the cluster until a certain volume is reached; and (iv) surrounding the cluster by a protecting shell that prevents agglomeration. Therefore, nanoparticles should be formed in the presence of a protecting agent. These protectors can be broadly divided into two categories: those providing electrostatic and those providing steric stabilization. The electrostatic stabilization is based upon the double electric layer formed when ions of the same sign are adsorbed at the nanoparticle surface. It is well known that the catalytic properties of heterogeneous catalysts are dependent on the particle size of the metal and the surface structure of the supports [19].Transition metal nanoparticles are effective catalysts for chemical transformations due to their large surface area and a unique combination of reactivity, stability, and selectivity. In most cases, the Controlling size and polydispersity of nanomaterials is a key requirement for most of their applications. PEG and PEO with different molecular weights find use in different applications and have different physical properties (e.g. viscosity) due to chain length effects, their chemical properties are nearly identical [20]. In transition metals no ble metals have high Standard reduction potential i.e.palladium metal having reduction potential 0.938 V [21]. Nanoparticles have been immobilized on inorganic solid supports [22] or embedded in organic polymers [23], dendrimers [24], multilayer polyelectrolyte films [25], and ionic liquids for separation and reuse [26]. However, the immobilization often suffers from problems such as low reactivity, degradation, palladium leaching, and difficult synthetic procedures. Very recently, reported a simple one-pot method of making recyclable palladium catalyst through generation of palladium nanoparticles from Pd(OAc)2 and Polyethylene glycol [27]. The use of simple and widely available polymers like PEGs as a non-toxic, inexpensive,non-ionic, thermally stable, recoverable, non-volatile polymers have been used for various transformations [28-33].Transition metal nanoparticles have wide ranging applications in catalysis. However, due to their large surface area and surface energy, they tend to agglomerate during the reactions and therefore need to be stabilized for effective utilization. In this work we described the synthesis and characterization of polymer PEG20K capped palladium nanoparticles of smallest size with low polydispersity
Experimental
Herein, we report a novel and facile route for preparation of Pd nanoparticles by exploiting PEG molecular weight 20000(M.W.20K), which was found to act as both reducing agent and stabilizer [27]. In the typical experiment a mixture of Palladium acetate Pd(OAc)2 (5.09x10-3M in 1,4-- dioxane ) solution and PEG, mol. wt. 20000 aqueous solution (2.0028%) in methanol (15 ml) were stirred at room temperature for 5 hours. Immediately after the two solutions were mixed, the solution became slightly yellow and slightly turbid, which indicated aggregate formation before the reduction of palladium ions. With course of time the color of the solution turned from orange to brown and finally turned black, indicating the formation of PEG20K capped Pd(0) metal nanoparticles (Scheme 1).
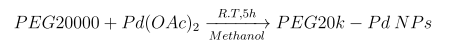
Scheme 1: Synthesis of polymeric PEG20K-Pd Nanoparticles
The mixing ratio of the PEG20K and palladium ions (PEG20K/ [Pd2+]) affected the formation of the spherical aggregates of the palladium nanoparticles. The reduction of Pd2+ ions followed an analogous polyol process in the current study [34].
When Pd(II) ions were added into the methanolic solution, electropositive palladium ions are rapidly trapped by electronegative oxygen forming weak metal ion complex followed by anlogus polyol process. In this system electron transfer between metal ions and the hydroxyl group leads to the reduction of Pd2+ to Pd (0). In sample 229-SG-6 (Table 1) it was found that the monodisperse smallest size PEG20K-Pd nanoparticles were obtained which was characterized by the DLS study (Figure 1a). The desired size and monodispersity was not obtained in rest of the samples (Table 1).In the standardization process to develop minimum size nanoparticles with high monodispersity only in the sample 229-SG-6 (Table 1) concentration ratio (0.6/0.4) of PEG20K to Pd(OAc)2 generates minimum size nanoparticles (less than 100 nm ) which was desired goal of this work. The experimental condition such as amount of protecting polymer, the concentration of the metal ions are systematically changed to achieve the smallest size PEG20K-Pd nanoparticles (Table 1; sample 229-SG-6).
Results & Discussion
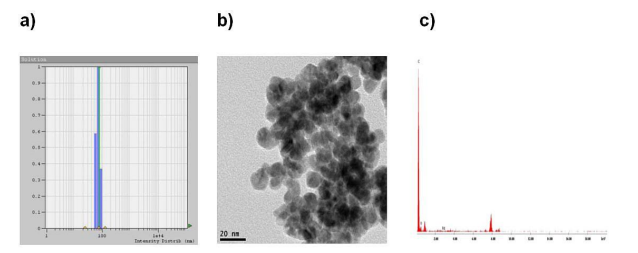
DLS evaluation (Table 1; sample 229-SG-6). of the nanoparticles indicates that the size distributions of the particles are very narrow. Sample 229-SG-6 found to be smallest size having polydispersity 0.242 (Table 1). We begin the studies with some preliminary investigations of the particle core size by using DLS (Figure 1a).Represented TEM images of PEG20K-Pd nanoparticles prepared using the chemical reduction method described in the experimental section is shown in Figure 1b.
Prepared nanoparticle possesses an average diameter of 13.0 nm and a standard deviation ±1 nm (calculated from the diameter of a sample of 40 nanoparticles); it is observed smaller monodispersed nanoparticle are obtained by chemical reduction method. The prepared PEG20K-Pd nanoparticles remained dispersed for several months with no obvious change in the size. The TEM analysis implies that the long chain structure of PEG could provide good stability and dispersing effects to the Pd nanoparticles and prevent its agglomeration. The composition of PEG20K-Pd was further probed by EDX analysis (Figure 1c). From the distribution of C, Pd, and O in the individual particles measured by EDX analysis, each element was non-uniformly distributed on the nanocomposites. These data gave a clue that the particles were nanocomposites consisting of Pd, C, and O. From the EDX spectra (Figure 1c) we can see that the nanoparticles are composed of C, O and Pd; this confirms the presence of palladium metal.
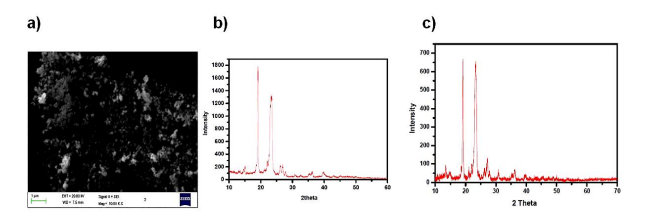
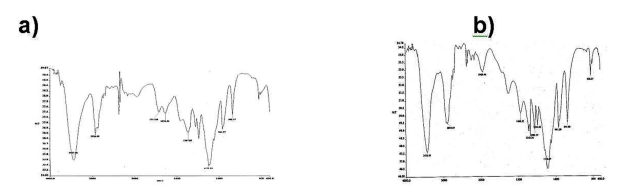
From the Figure 2a. it is obvious a large amount of sample is dispersed on the slide .The average grain size was found to be 100 nm with round morphology. Agglomerated grain were attached on the polymer surface .The surface property was found out to be distorted, rough without any specific pattern. The formation of nanoparticles is confirmed by observation of broad peaks in the XRD spectrum (Figure 2b). Reflections due to (111) and (200) planes at 2θ=39.64, and 45.62 confirmed the presence of palladium metal in the nanoparticles. EDX data (Figure 1c) shows the high content of carbon (98.5% by atomic weight) in the prepared nanoparticles. Presence of high carbon content shows that prepared PEG20K-Pd nanoparticles in the obtained powdered XRD (Figure 2b) are highly amorphous in nature. Pure PEG20K (Figure 2c) shows that the diffraction peaks between 2θ = 19.16º, 23.24º, 27.2º, 30.76º and 36.25º . The peaks corresponding to the polymer still appears in the XRD pattern of prepared PEG20K-Pd nanoparticles (Figure 2b). Compared with the pure polymer PEG20K, PEG20K–Pd nanoparticles (Figure 3a) showed a new broad band at 2000 cm-1, which is assigned to presence of Pd(0)–O compounds [35]. It is observed that there is a increase in the IR frequency of pure ether linkage peak at 1123.55 cm-1 (Figure 3a) bonded to the palladium metals. The methylene antisymmetric and symmetric vibration modes at 2926.98 and 2850 cm-1, respectively, are clearly seen in Figure 3a and indicate that the hydrocarbon chains capping the palladium nanoparticles are closely packed without a significant density of defects in the chains [36]. A broad peak centered at 3437.56 cm-1is observed and is assigned to the O–H stretch modes of vibration from the traces of uncoordinated PEG20K molecules remained [36]. In addition, PEG20K-Pd nanoparticle (Figure 3a) more bands at 2926.28 and 1367.83 cm-1 corresponding to the vibration modes of methylene group C-H stretching and OH bending modes. The absorption around 1468.53 cm-1 (Figure 3b) is due to bending vibration of -CH2 group which was found to be disappeared in the PEG20K-Pd nanoparticles (Figure 3a). A sharp, strong band at 961.77 cm-1and 842.17 cm-1 is due to the CC stretching vibrations (Figure 3a) [37]. The above analysis establishes the formation of the polymer metal interaction.
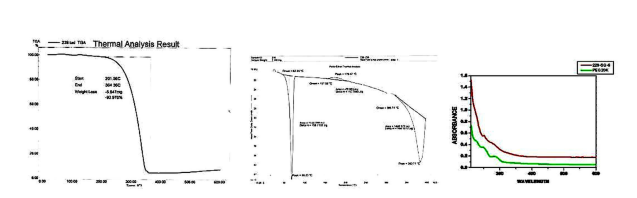
The palladium nanoparticles are capped with PEG20K molecules that provided sufficient hydrophobicity to the nanoparticles. These nanoparticles are stable up to several months at room temperature. To understand the stability of these systems at higher temperatures, thermo gravimetric analysis was performed on purified samples. The TGA profile for PEG20K-Pd system is shown in Figure 4a. Prominent losses occur between temperature range 201.56 and 364.36 °C, accounting for a total loss 93.976% which is due to desorption of PEG20K from the surface of the nanoparticles.
However the weight loss found (93.976%) seems to be large for decomposition of PEG20K monolayer formed at Pd nanoparticle surface [38]. This indicates that the observed weight loss would include loss of not only PEG20K bound to Pd particles, but also unbound PEG20K molecules and traces of solvent molecules. The presence of two distinct temperatures at which weight loss occurs indicates the possibility of two different modes of binding of the PEG20K molecules with the palladium nanoparticle surface. Figure 4b shows the enthalpies of transitions of PEG20K-Pd nanoparticles. A sharp peak at 68.25 ⁰C and enthalpy 156.7132 J/g shows the melting of polymer (Tm). Second peak around 179.47 ⁰C having enthalpy 78.803 J/g shows the separation of the polymer from the Palladium metal from one binding site. Third peak (366.74 to 383.71 ⁰C) having enthalpy 1446.577 J/g shows the complete separation and degradation of polymer from the entire binding site from the metal. It concludes that as the temperature increases, the state of polymer changes from solid to liquid and finally breakdown of the polymer chain take place. The UV–Vis spectrum of PEG20K stabilized palladium shows absorption maximum at around 272 nm, (Figure 4c) which is characteristic of prepared PEG20K–Pd nanoparticles. From the UV–Vis spectra it is concluded that PEG has capped the palladium metal which is also supported by the TGA data.
Conclusion
The utilisation of nanodimensional materials offers significant benefits in a range of different applications. In order to maximise their usefulness, reliable synthesis are required that can generate well-defined nanoparticles with a high degree of monodispersity. This aim was being achieved in the synthesis of PEG20K-Palladium nanoparticles by using polyethylene glycol 20,000 sterically bulky molecules to control the synthesis and get smallest size nanoparticles with high monodipersity (Table 1; sample 229-SG-6). This enables properties such as the size, shape, solubility and surface functionality of the resulting nanoparticles to be carefully tuned. Such materials are being explored for many different applications, especially in catalysis, where palladium can effectively catalyse a range of different transformations.
Author Contributions
SG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - review & editing, Writing - original draft.
DTM: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation.
RK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Funding acquisition, Supervision, Validation, Visualization.
Conflicts of Interest
There are no conflicts to declare.
Acknowledgements
We are pleased to acknowledge financial support from UGC No.F.30.-109/2015 (BSR) & DST Order No. SR/FT/LS-58/2012 for this investigation. We are also thankful to Department of Chemistry, University of Delhi, Delhi, India and USIC of Delhi University for providing technical support.
- Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology, Chem. Rev. 104: 293-346.
- Henglein A (1989) Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles Chem. Rev. 89: 1861-73.
- Templeton AC, Wuelfing WP, Murray RW (2000) Monolayer-protected cluster molecules, Acc. Chem. Res. 33: 27-36.
- Schmid G, Corain B (2003) Nanoparticulated Gold: Syntheses, Structures, Electronics, and Reactivities, Eur. J. Inorg. Chem. 17: 3081-98.
- Lewis LN (1993) Synthesis, Structure, and Physicochemical Properties of [Mo6Cl8]4+-Containing Clusters Chem. Rev. 93: 2693-730.
- Bradley JS (1994) The Chemistry of Transition Metal Colloids. In Clusters and Colloids, From Theory to Applications; Schmid, G., Ed.; VCH: Weinheim, Germany, 459-544.
- Braunstein P, Oro L, Raithby PR (1998) In Metal Clusters in Chemistry;: Weinheim, Germany.
- In Nanoparticles and Nanostructured Films (1998) Preparation, Characterization and Applications; Fendler, J. H., Ed.; Wiley-VCH:Weinheim, Germany.
- Klabunde KJ, Mohs C (1998) Nanoparticles and Nanostructural Materials. In Chemistry of Advanced Materials. An Overview; Interrante, L. V., Hampden- Smith, M. J., Eds.; Wiley-VCH: New York, 7: 271-327.
- Aiken III, JD Finke (1999) A review of modern transition-metal nanoclusters: their synthesis, characterization, and applications in catalysis, J. Mol. Catal. A. 145: 1-44.
- Rao CNR, Kulkarni GU, Thomas PJ (2000) Metal nanoparticles and their assemblies, Edwards, P. P.; Chem. Soc. Rev. 29: 27-35.
- Horn D, Rieger J, (2001) Organic Nanoparticles in the Aqueous Phase-Theory, Experiment, and Use, Angew. Chem., Int. Ed. Engl. 40: 4330-61.
- a) Reetz MT, Winter M, Breinbauer R, Thurn-Albrecht T, Vogel W, Chem. Eur. J. 2001, 7, 1084- 94.b) Harraz FA, El-Hout SE, Killa HM, Ibrahim IA, J Catal.2012, 286: 184-92. c) Arvelos MS, Silva AC, De Souza ALF, Achete CA, Vasconcelos TL, Robertis E, Archanjo S, B.; A,; LCS Malta, LFB, Senra, J. D. Chem. Sel. 2018, 33: 9725-30.
- Caruso F (2001) Nanoengineering of Particle Surfaces, Adv. Mater, 13: 11-22.
- Bo¨nnemann H, Richards RM (2001) Nanoscopic Metal Particles − Synthetic Methods and Potential Applications, Eur. J. Inorg. Chem, 2455-80
- Rao CNR, Kulkarni GU, Thomas PJ (2002) Size-dependent chemistry: properties of nanocrystals, Edwards, P. P.; Chem. Eur. J. 8: 28-35.
- Feldheim DL, Foss CA, (2002) Metal nanoparticles: synthesis, characterization, and applications, Jr., Eds.; Marcel Dekker: New York. Chapter 2: 17
- Roucoux A, Schulz J, Patin H (2002) Reduced Transition Metal Colloids: A Novel Family of Reusable Catalysts? Chem. Rev, 102: 3757-78.
- (a) Feldheim, D. L.; Foss, C.A. Jr. Metal Nanoparticles: Synthesis, Characterization, and Application; Marcel, D. New York, 2002. (b) Stiles, A.B. (ed.) Catalyst Supports and Supported Catalysts; Butterworths: Boston, 1987. (c) Ertl, G.; Knozinger, H.; Weitkamp, J. (eds.) Handbook of Heterogeneous Catalysis; VCH: Weinheim, 1997. (d) Verma, A.K.; Kumar, R.; Chaudhary, P.; Saxena, A.; Shankar, R.; Mozumdar S.; Chandra, R. Tetrahedron. Lett, 46: 5229-32.
- Thesis awarded topic entitled: Preparation & Characterization of Palladium Nanoparticles Using Organic Molecules. Gupta; S. Department of Chemistry, University of Delhi, Delhi (india).
- Keith F, Purcell, John C, Kotz (1977) Inorganic chemistry, Philadelphia : Saunders.
- Choudary BM, Madhi S, Chowdari V, Kantam ML, Sreedhar B (2002) J. Am. Chem. Soc, 124: 14127–36. (b) Djakovitch L, Koehler K (2001) J. Am. Chem. Soc. 123: 5990-9.
- Chauhan BP, Rathore JS, Chauhan M, Krawicz A (2003) J. Am. Chem. Soc, 125: 2876-87. (b) Biffis A, Zecca M, Basato M (2001) J. Mol. Catal. A: Chem, 173: 249-74.
- Ooe M, Murata M, Mizugaki T, Ebitani K, Kaneda K (2004) Epoxidation of alpha,beta-unsaturated ketones using hydrogen peroxide in the presence of basic hydrotalcite catalysts, J. Am. Chem. Soc, 126: 1604-5.
- Kidambi DJ, Li J, Bruening ML (2004) J. Am. Chem. Soc, 126: 2658-9.
- (a) Huang J, Jiang T, Gao H, Han B, Liu Z, Wu W, Chang Y, Zhao G (2004) Angew. Chem.; Int. Ed, 43: 1397-9. (b) Dupont, J.; Fonseca, G.S.; Umpierre, A.P.; Fichtner, P.F.P.; Teixeira, S.R. J. Am. Chem. Soc, 124: 4228-9.
- Ahmadian Namini P, Babaluo AA, Bayati B (2007) Palladium nanoparticles synthesis using polymeric matrix: poly (ethyleneglycol) molecular weight and palladium concentration effects IJNN, 3: 37-43.
- (a) Kumar, R.; Chaudhary, P.; Nimesh, S.; Verma A.K.; Chandra, R. Green Chem. 2006, 8, 356–358. (b)Harris, J.M. (ed.) Poly (ethylene Glycol) Chemistry, Biotechnological and Biomedical Applications; Plenum Press: New York, 1992, 41, 233–234; Polyethylene Glycol: Chemistry and Biological Application, ACS Books, Washington, DC, 1997. (c) Yadav, V.; Lagarkha, R.; Kumar, R. Asian J. Chem. 2009, 21, 5591–5597.
- Sauvagnat B, Lamaty F, Lazaro R, Martinez (1998) Chemical reactivity in matrix-assisted laser desorption/ionization mass spectrometry, J Surf Chem. Catal. 777-82.
- Chandrasekhar S, Narsihmulu C, Sultana SS, Reddy NR (2002) Osmium Tetroxide in Poly(ethylene glycol) (PEG): A Recyclable Reaction Medium for Rapid Asymmetric Dihydroxylation under Sharpless Conditions. Org. Lett. 4: 4399-401.
- Jian, S.Z.; Wang, Y.G. Chem. Lett. 2004, 33, 866–867.
- Xia M, Wang YG (2002) Tetrahedron Lett, 43: 7703-5.
- Wang YG, Zhang J, Lin XF (2003) Cross-Electrophile Coupling: Principles of Reactivity and Selectivity, Synlett, 10: 1467-8.
- Li H, Jo JK, Zhang LD, Ha CS, Suh H, Kim I (2010) Methylammonium Chloride Induces Intermediate Phase Stabilization for Efficient Perovskite Solar Cells, Langmuir, 26: 18442-53.
- Anema JR, Li JF, Yang ZL, Ren B, Tian ZQ (2011) Shell-isolated nanoparticle-enhanced Raman spectroscopy: expanding the versatility of surface-enhanced Raman scattering, Annual Review of Analytical Chemistry, 4: 129-50.
- Hostetler MJ, Stokes JJ, Murray RW (1996) Infrared Spectroscopy of Three-Dimensional Self-Assembled Monolayers: N-Alkanethiolate Monolayers on Gold Cluster Compounds, Langmuir, 12: 3604-12.
- P Anji Reddy, K Ranveer (2011) Conductivity, XRD, and FTIR studies of new Mg2+-ion-conducting solid polymer electrolytes: [PEG: Mg(CH3COO)2], Journal of the Korean Physical Society, 59: 114-8.
- K Ashavani, M Saikat, SPR P Renu, MAB, S Murali (2003) Investigation into the Interaction between Surface-Bound Alkylamines and Gold Nanoparticles Langmuir, 19: 6277.

Tables at a glance
Figures at a glance