Designing Efficient Plasmonic Biosensors Based on Gold Metallic Nanostructures
Received Date: February 27, 2023 Accepted Date: March 27, 2023 Published Date: March 31, 2023
doi: 10.17303/jnsm.2023.9.101
Citation: Genene Gebremedhin, Alemayehu Nana Koya (2023) Designing Efficient Plasmonic Biosensors Based on Gold Metallic Nanostructures. J Nanotech Smart Mater 9: 1-14
Abstract
The increasing need for plasmonic bio-sensing devices that require analytical platforms which are efficient, instant, extreme sensitivity, and real-time response, have yielded a significant change in the design them in recent years. The development of sensors based on plasmonic nanostructures has exhibited the best quality approach to integrate them in the lab-on-chip platforms with miniaturization and multiplexing. The main goal of this study was to design a highly sensitive nano-sensor based on metallic nanostructures and to investigate the effect of size and shape on the optical properties of metallic nanoparticles in very large spectral range (λ =500–1200 nm) using simulation for nanoparticles of sizes (D = 100–200 nm). In particular, the optical properties of gold nanoparticles were investigated using the Finite-Difference Time-Domain (FDTD) method. The wavelength corresponding to the maximum scattering redshifts (shift to longer wavelengths) were observed as the nanoparticle size increased. The influences of Au NP size and shape were analyzed in detail. The gold nanoparticle diameter between 100 nm to 200 nm is used in determine the shifting of the surface plasmon resonance. The results in this work indicated that the position of the plasmon resonance wavelength for gold nanoparticle was redshift when the size of gold nanoparticle was increased.
Data was collected after designing the simulation of nanoplasmonic structures using Finite-Difference-in Time-Domain (FDTD) software. The major finding showed that adjusting of Au nanoparticles sizes/diameters and varying the sensing environment enhanced the resonance wavelength shift; this increased the sensitivity of Au nanoparticles. This study offers a new insight regarding biosensors based on plasmonic nanoparticles and it will provide opportunities for developing Plasmon-enabled applications in biomedicine.
Keywords: Plasmonic Biosensors; Gold Metallic Nanostructures; Plasmonic Nanostructures
Introduction
Overview of Plasmonic Biosensors
Optical biosensors show unquestionable advantages compared to other biosensors since they can deliver label-free quantitative analysis and show exceptional potential for multiplexing and miniaturization. Plasmonic biosensors can be classified into two; those that use thin metallic films and those that use individual inorganic Plasmon resonant nanostructures.
By far the most widely used type of plasmonic biosensor is known to most people simply as ‘surface Plasmon resonance (SPR), a film-based sensor that has become the 'gold standard for characterizing the interaction between biomolecules. Since its use in real-time analysis of a biological systems in 1990s SPR has become an important optical bio-sensing technology in the areas of biochemistry, biology, & medical sciences because of its real-time, label-free, and noninvasive nature. Commercial SPR devices are prohibitively expensive and require consumable sensor chips that fit certain specification of size, thickness, and so forth. For example, Biacore (acquired by General Electric Health care in 2006) provides several models of SPR-based instruments (cost$120,000-$250,000) that are compatible only with expensive Biacore accessories and consumables (electrode chips cost$60-$120/each). [38] SPR bio-sensing appears to be one of the most powerful approaches monitoring of affinity binding of molecules, and primary screening of drug gable molecules. SPR –type sensors are increasingly used to study variety of biological entities, such as DNAs, RNAs, proteins, carbohydrates, lipids, and cells in the field of biomedical research [39].
Additionally, those based on localized surface Plasmon resonance (LSPR) have been subjected to a great scientific interest the last few years as the novel counterpart of the well-established SPR sensor. [1]
Propagating plasmonic sensors depend on the surface plasmons resonance (SPS). Collective oscillations of free electrons are supported by thin (~50nm) metallic films. Gold is a common film since it produces SPRs that can be interrogated using visible wavelengths of light, it is a relatively inert metal (not prone to oxidation), and it can be easily functionalized with molecular linkers through favorable gold-thiol interactions or adsorption. Excitation of the SPR in film can be done in several ways, with the most common being the so-called Kretschman configuration[2,3] where light is coupled into the gold film by the glass prism that facilitates total internal reflection (TIR) of the input beam at the gold film /air interface. However, an alight coupling mechanism is necessary to match the momentum of the incoming photons to the free surface electrons of the gold film and create an SPR.
Film SPR experiments can be classified as angle-resolved or wavelength-length-resolved SPR, and are differentiated by the type of source used and hence the type of data that is gathered.
The basis of the film SPR sensing mechanism is the fact that the SPR is highly sensitive to the refractive index (RI) of the medium in direct contact with the metal film, which in the case of biosensing is usually an aqueous solution (watch, buffer, etc.).
As is the case in Nanoscale thin metal films, metal nanostructures that are significantly smaller than the wavelength of incident light upon them can exhibit an SPR or a collective of the free surface electrons in response to the oscillating electric field of the light. When SPRs are excited in Nanoscale 3D objects, and hence are called localized surface Plasmon resonances (LSPR). Given that LSPR signal is generated from Nanoscale objects, LSPR sensing platforms are more amenable to multiplexing and miniaturization than film SPR sensing platforms. This makes LSPR plasmonic sensors an attractive option for incorporation into miniaturized lab-on-a-chip (LOC) or point-of-care (POC) diagnostic devices
A LOC is a device that integrates several analytical functions on a single chip only a few millimeters up to centimeters in size. It is an automated miniaturized laboratory system used for different clinical applications inside and outside the hospital. Examples of applications include measurements of blood gases, blood glucose, and cholesterol or counting the number of HIV cells. Making use of microfludics-the technology to control and handle small volumes of liquids-analytical processes is miniaturized to enhance mobility and efficiency. This makes LOC applications suitable for clinical diagnostics and ‘near-patient’ or ‘point-of-care POC tests. [40]
APOC is a portable device used for analysis and detection outside a conventional laboratory. A point-of-care test (POCT) is a simple analytical test that can provide patients with a medical diagnosis in real-time, even in environments with limited resources. The application of POCTs has brought hope to developing countries with limited infrastructures and economic development but that still require timely medical detection services. [41]
Optical Properties of Metallic Nanoparticles
Nano materials are macroscopic systems but constructed and organized from elementary bricks of Nano metric dimensions known as nanoparticles. When the dimensions of a material are reduced to nanometer scales almost all of its properties change. There are two main types of confinements which playa strong role in determining the optical properties of metal particles, namely dielectric and quantum confinements. When dimensions of the particle are smaller than the mean free path of the electrons the energy level of structure for the particle changes. This change shows up in various optical, electrical and thermodynamic properties of nanoparticle. Such modifications are due to quantum nature of charge carriers confined inside the metal particles, and are called quantum confinement effects. The metal particles are usually embedded inside a host which has different dielectric properties. The difference in the dielectric constants between the host and metal particles leads to change in the electromagnetic field distribution inside and around the particle. This leads to what is known as the dielectric confinement. Localized surface Plasmon resonance (LSPR) is one of the manifestations of the dielectric confinement. The dielectric confinement and hence the LSPR of the sample depends on shape and size of the particle. Particles with sizes in the range of 1-100 nm are called nanoparticles, whether they are dispersed in gaseous, liquid, or solid media [32-33]. In size, it goes from around 1 nanometer to 100 nanometers. In shape, it can be either isotropic like sphere, or anisotropic like rod, wire, cube, prim, star, or even amorphous. They have many fields of application, in the fields of optics [25,26], magnetism [27,28], electronics [29,30], and telecommunications [31]. Because the NPs are larger than individual atoms and molecules but are smaller than the bulk solid, materials in the nanometer size regime show behavior that is intermediate between that of a macroscopic solid and that of an atomic or molecular system. The properties of materials with nanometer dimensions are significantly different from those of atoms and bulk materials. There are three major factors that are responsible for these differences: high surface-to-volume ratio, quantum size effect and electro dynamic interactions.
All nanoparticles regardless of their chemical constituents have surface area to volume ratios that are extremely high. One of the direct effects of reducing the size of materials to the nanometer range is the appearance of quantization effects due to the confinement of the movement of electrons.
Among different nanomaterial employed in research, metallic NPs have been proved to be the most convenient and suitable. Metallic NPs possess unique optical, electronic, chemical and magnetic properties that are strikingly different from those of the individual atoms as well as their bulk counterparts. It is known that the intrinsic properties of metal NPs are mainly governed by their size, shape, composition, crystallinity and structure. If such tiny particles are allowed to coalesce in a controlled fashion, their color can be systematically varied from pink through violet to blue [34,35]. The dimension of the particles in the nanometer size regime makes them ideal candidates for Nano engineering of surfaces and the fabrication of functional nanostructures [36,37].
Nanoparticles are interesting to researcher for number of different reason. They have a large surface area to volume ratio which means they have a large surface area to take part in chemical interaction; this is an excellent property for catalysis. Metal nanoparticles are very attractive because of their size and shape dependent properties. There are so many metallic nanoparticles like Ag, Cu, Au, lead, platinum etc. Gold nanoparticles (AuNPs) are a popular choice of nanomaterials for many researchers to work with because of their facile methods of synthesis, high degree of control over shape and size and long-term stability in a wide range of solvents.
Even though Gold nanoparticles are the object of intense research because of their fascinating Nano scale behavior. Colloidal gold nanoparticles are particularly interesting because of its easy preparation and high stability. These metals display bright colors when particles of sizes of a few to a few hundreds of nanometers are dispersed in a medium or on a surface. Among the metallic nanomaterial’s gold nanoparticles are particularly interesting due to their easy methods of synthesis, the ability to adjust properties through size and shape and their stability in a wide variety of solvents conditions. However, through research, the size, shape, surface chemistry and optical properties of Au NPs are the parameters which are under control and have opened new doors to some very unique and exciting capabilities.
Engineered plasmonic materials have defined chemo-physical properties, and use in a series of state-of-the art applications with unique mechanisms. Typically, the design of plasmonic materials requires consideration of composition, morphology, and surface chemistry, with regard to composition; noble metals (e.g. Au and Ag) are the most used candidates for desirable surface plasmon. With regard to surface chemistry, specific molecular capture and recognition. Moreover, engineered plasmonic materials should be integrated with a device of assays. To date, advanced spectrometric methods require the rational design of plasmonic materials that address all the above considerations, which require inter-disciplinary research efforts towards application in the diagnostics field. The unique opto-electronic properties of plasmonic nanomaterials impart characteristics features based on their size, shape and composition. The controllable size and shape of these particles can be tailored by optimization synthetic conditions, such as stabilizing agents, surface fictionalization and temperature variations [42].
Among the nanomaterials currently under exploration, gold nanoparticles (AuNPs) and their colloidal dispersions are promising candidates for future scientific, industrial, and domestic applications. AuNPs have a rich history, dating back to ancient Roman times when they were used to stained glass for decoration purposes. The modern era of AuNPs synthesis began over 150 years ago with the work of Michael Faraday, who was the first to observe that colloidal Au solutions exhibited properties that differ from bulk Au, but it was not until 1908 that the color effects associated with colloidal AuNPs were rationalized by Gustav Mie. The intense red color of AuNPs is now known to be caused by the interaction of incident light with the free electrons in the particles, creating a collective oscillation of electrons, the so-called localized surface plasmon resonance (LSPR). AuNPs with different diameters in the range of about 10–100 nm or AuNPs aggregation produces color changes from red to blue. Colorimetric sensors based on AuNPs have been widely explored and shown promising applications. In contrast to the LSPR-active nanoparticles, ultras- mall AuNPs (typically 2 nm diameter) no longer support the LSPR excitation due to quantum size effect. Such ultra- small particles are often called Au nanoclusters (AuNCs) to differentiate them from their larger counterparts – plasmonic nanoparticles, typically 2 nm). Owing to the ultra-small size, AuNCs exhibit discrete electronic structure and distinctive properties that are fundamentally different from those of metallic nanoparticles. AuNCs have received considerable research interest due to their strong fluorescence in the visible and near-infrared (IR) spectrum for promising applications in sensing and biological events. The discovery of AuNCs photoluminescence was reported earlier, stating that luminescence should intensify with decreasing particle size. This transition can be seen where LSPR of AuNPs diminishes and photoluminescence occurs in the AuNCs size regime.
In recent years, AuNPs have been functionalized using various methods, and they have been the focus of extensive research activities. Functionalized AuNPs have been extensively studied in analytical applications. Although there have been many review articles published in the past on the use of AuNPs in biological and chemical applications, we feel it is time to write an updated review to discuss the most recent developments in analytical application, especially in chemical and biological sensing [43].
Statement of the problem
Film-based SPR studies showed that excitation of the SPR by using the Kretschman configuration has advantages and disadvantages [1]. The advantage is the optical path of the sensing platform is contained on the backside of the sensor surface, which facilitates the use of flow cells on the top of the sensor surface
Because the SPR sensitivity to refractive index (RI) only extends ~200nm into solution, SPR sensing is an interfacial sensing technique, meaning that it only detects RI changes that happen close to the metal film surface.
An important advantage of film SPR studies is the fact that the binding interactions can be studied in a label-free format, meaning that once the capture molecule is immobilized onto the sensor surface, no modifications are necessary to detect the binding events that occur on the surface. Though film SPR is a widely established, robust, and commercially available tool for studying interfacial phenomena, the instrumentation required for firm SPR analysis can be considered bulky from the standpoint of miniaturized sensing devices. This is due to the optics required to couple light into the metal firm to excite the SPR, the need for temperature control to produce a stable SPR signal, and also to the propagation length of the firm SPR laterally on the firm surface (from few to hundred if micrometers [4], which sets the lower limit on the size of the sensor surface [4].
For these reasons, it is generally difficult to incorporate firm SPR sensors into small, portable, and multiplexed sensing devices that would perform in a lab-on-a-chip setting. That said, however, firm SPR studies can exist in a multiplexed format by multichannel SPR instrument or SPR imaging [5-7].
LSPRs of Nanoscale objects can be tuned by varying the size, shape, and composition of nanoparticles (NPs) [8,9]. This is advantageous for sensing in biological samples because detection systems can be tuned to use wavelengths that do not overlap with the spectral features of strongly absorbing naturally occurring biological chromo spores. Such as hemoglobin in blood samples, to improve sensitivity to target analytes.
LSPR sensors are similar to film SPR sensors in that they are very sensitive to the RI of the surrounding medium, however, LSPR sensors have dramatically reduced sensing volumes [4,10] and produce more localized sensor information.
Though Single NP LSPR sensors approach single-molecule sensitivity, are label-free, and perform similar to gold standard film SPR platform, the largest drawback of their use is the cumbersome nature of the experimental setup, so a dark field microscope with the attached spectrometer is needed, and also an interrogation of the single NPs is usually done one-by-one (though this is improving with various optical and imaging techniques [11,12-14]), so it becomes time-consuming to gather enough data for statistical relevance in high throughput.
Biosensor devices provide the possibility to create miniaturized tools to containing some of the functionalities of an entire analytical laboratory; this fact has increased scientific research during the last decades. Despite the recent technological achievements, the transition from proof–of–concept devices at a laboratory scale to real application in the field with a tangible impact on the research and industry is often lacking, on the other hand, this may be correlated to the fact that most of the recent biosensing platforms are based on complex and expensive fabrication methods with bulky detection schemes, limiting their mainly to laboratory scale. Fabrication methods with bulky detection schemes, limiting their mainly to laboratory scale.
In this context, simple nanostructure-based plasmonic biosensing platforms represent a highly attractive tool for multiplexed detection and the development of portable point-of-care (POC) platforms in a cost-effective manner. Under this assumption, the main objective of this paper will focus on developing a novel plasmonic biosensor based on LSPRs Nps as a substrate containing to generate Plasmon resonance by coupling them with different metallic layers on a Nano metric scale. The final scope is the integration into a lab-on-a-chip (LOC) platform for real-time and simultaneous detection of different biomarkers in human fluids. Examples of ready-to-use is Nanoparticle Tracking Analysis(NTA) which utilizes the properties of both light scattering and Brownian motion in order to obtain the Np size distribution of samples in liquid suspension and MEMS sensor is under use [46].
Despite increased research reports on the optical properties of metallic nanoparticles, there are several open questions regarding optical properties of metallic Nano particle that need to be answered. One of the active research topics of the effect of size and shape on optical properties of metallic nanoparticle is accurate prediction of the optical response of metallic nanoparticles as a function of their size and shape.
Although gold has interesting optical properties, its potentials in metallic properties have been extensively investigated only as spherical particles. Further when gold is in spherical form offers limited opportunity to tune optical properties through variation in size and shape. This objective can be achieved through changing the morphology of gold nanoparticles from conventional spherical particles. Particularly, gold nanoparticles have interesting optical responses when their shapes and sizes are altered. To this end, in this study, the optical responses of gold nanoparticles with different sizes and shapes have been investigated. Specifically, an Au Nano cone is systematically morphed into equivalent Nano cylinder, both having the same base and height. Based on numerical results the near-field and far-field optical properties of such metallic nanoparticles have been predicted.
Thus, in this study we investigated the sensing performance systematically designed nanosensor and explore optical responses of Gold nanoparticles with different sizes and shapes. Particularly, Au nanoring and Au Nanodisc particles with variable geometry and mediums. In addition, Au Nano cones into equivalent Nano cylinder both having the same base and height. We showed the effect of NP size and geometry on plasmonic response and enhanced its refractive index sensitivity. Finally, we have carried out a detailed study of the spectral responses and local field properties of systematically designed nanosensor based on metallic nanostructures. Base on numerical results the near-field and far-field optical properties of such metallic nanoparticles predicted.
Evaluating Nps in range of 100nm to 200nm size impacts distribution within specific organs, as well as affecting the bio-distribution throughout the body. Nps need to reach the targeted organ or tissue in order to realize the desired function. These gold Nps have spherical shape completely soluble in water; have been used widely for the development of diagnostic probes. These Au Nps can be further modified with different surface coating and functional groups to render them with unique chemical and biological reactivity [44-45] In recent years, most Nps have been designed to be 100nm to 200nm in size since that small particle Np helps to extend half-life of the drug and improve the penetration efficiency of the drug [46]. This research aims to illustrate top-down strategies for nanomaterial specification of instrumentation using FDTD software [47,48].
The general objective of the study
The main goal of this study was to design highly sensitive plasmonic biosensors based on metallic nanostructures and to investigate the effects of size and shape on optical properties of metallic nanoparticles.
Specific objective of the study
The specific objectives of the study were:
To design an efficient plasmonic nanosensor
To explore the optical properties of systematically designed nanosensor.
To check the performance of plasmonic nanostructure based on biosensing platforms
To investigate the optical properties of metallic Nano particles.
To theoretically study the effect of size on the optical responses of nanoparticles.
To explore the effect of shape on the optical responses of nanoparticles.
To study the spectral shift as functions particle size and shapes
Methods
Nanomaterials have structures sizes smaller than 100nm in at least two dimensions. These Nanomaterials can have various shapes and structures such as spherical, needle-like, tubes, platelets, etc. Chemical composition is another important parameter for the characterization nanomaterials, which comprise nearly all substance, classes e.g. Metals/ metal oxides, polymers, compounds as well as biomolecules [49].
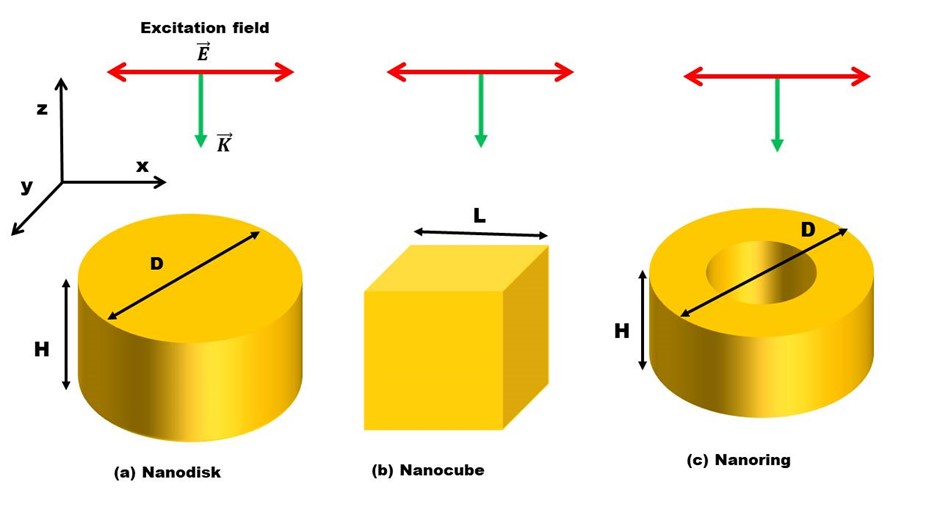
A schematic representation of the studied nanostructures is displayed in Figure 1. Initially, we used gold nanoparticle discs and rings of sizes ranging from 100nm to 200nm presented. The designed nanoparticles were illuminated from the top with a linearly polarized plane wave. To calculate the near-field and far-field responses of Au nano disk particles various sizes (see F2a) and to analyze the shape effect on the optical response, both the near-field profiles and far-field spectra of the nano cube and nano ring particles displayed in Fig 2b and c. Incident light is illuminated on nanoparticles of various sizes and shapes (Nanodisc, Nano cube and Nano ring) from top excite localized surface plasmon resonances. The optical responses of the designed nanostructures were calculated using FDTD method.
Result and Discussion
For characterization of Au Nps transmission electron microscope (TEM) used to characterize Nps to gain information about particle size, shape and inter-particle interaction. TEM is a high spatial resolution structural and chemical characterization tool.
Optical properties of a single Au Nano disk (ND) with different sizes
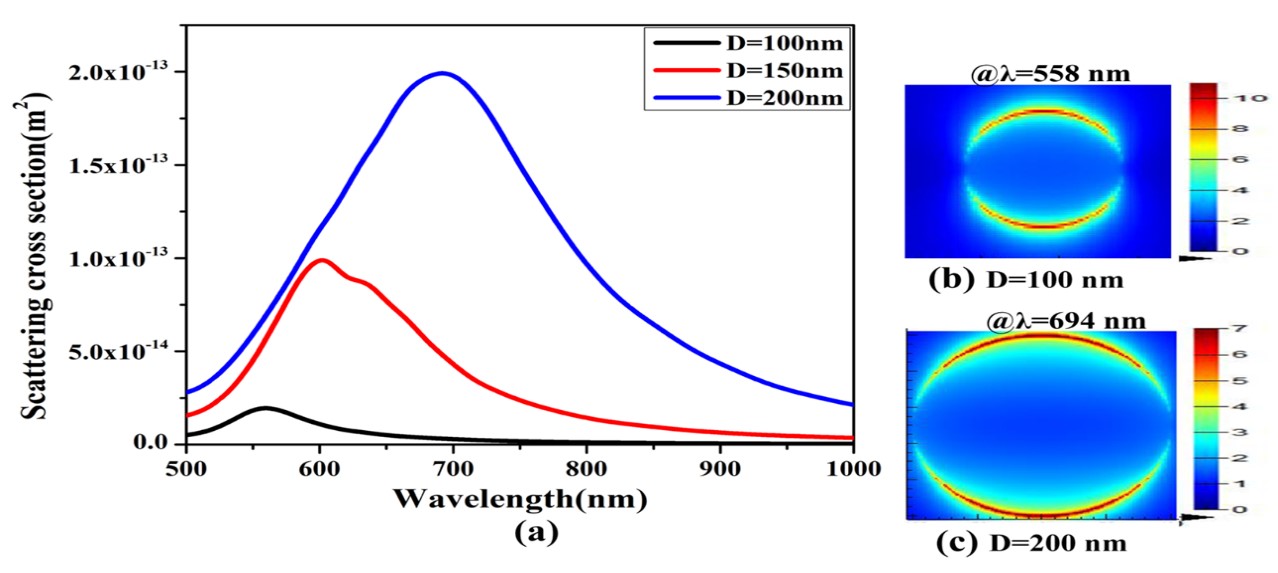
Figure 3(a) shows that the simulation result of Au ND with sizes/diameters D=100nm, 150nm, and 200nm respectively. The scattering cross-section here redshifts as the size of Au ND increases. The figure also discloses the resonant near-field properties of the Nanodisc particle as a function of its size. Figure3 (a) indicates the resonance wavelength of Au ND with D=100nm and 200nm are 558 and 694nm respectively. In addition it implies that the maximum resonance shifts in wavelength (∆λ ) is 136nm as the size of it changes from 100 to 200nm. Moreover, the scattering cross-section of Au ND changes from 1.99×〖10〗^(-14) m^2to19.9×〖10〗^(-14) m^2, as the size ND size increases from D=100nm to D=200nm. That is, as the size of Au ND increases the scattering cross-section increased ten times to the earlier. This is further supported by the local field profiles displayed in Figure 3(b) and3(c).
In the same fashion, the near-field profiles displayed in Figure 3(b) and (c) for various Nano disk sizes imply striking difference. As the scattering intensity increases with the size of the Nano disk, the intensity of the local fields calculated at resonant wavelengths of the Nano disk with 100 nm and 200 nm diameter show significant variation in intensities (compare Figure 3(b) and (c)).
Optical properties of a single Au nanoring with different sizes
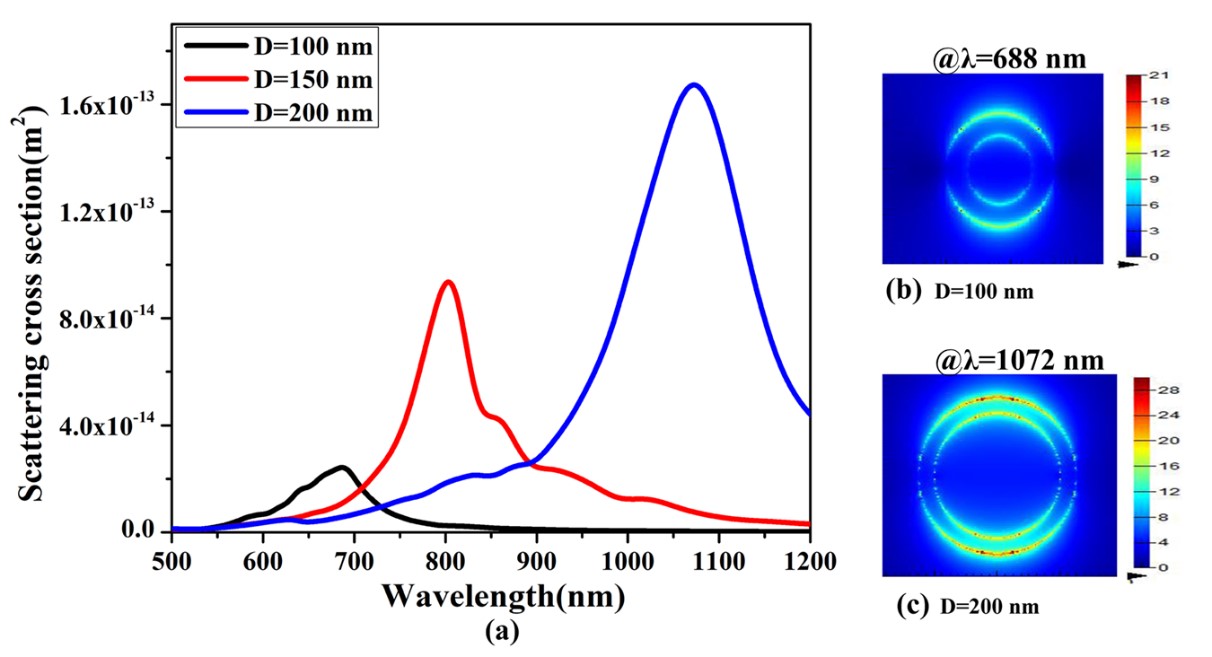
Figure 4(a) indicates the scattering of a single Au nanoring (NR) as a function of the NR diameter (D=100nm and 200nm). So the scattering resonance wavelength tends to redshift as the NR size increases. Moreover, the scattering cross-section of Au NR increases from 2.44×〖10〗^(-14) m^2to 16.8×〖10〗^(-14) m^2, as the NR diameter increases from 100nm to 200nm. Hence, it is possible to get enhanced LSPR in the region. Fig 4(b) and4(c) indicate the corresponding near-field profiles calculated at resonant wavelengths of the NR with sizes of D=100nm and D= 200nm respectively. This further confirms the effect of nanoparticle size on the plasmonic responses. The resonance wavelengths of Au Nano ring (NR) with diameter D=100 nm and 200 nm are 688 nm and 1072 nm, respectively (see Figure 4(a)). The resonance shift (Δλ) is 384 nm. The scattering cross section of Au NR with D=100 nm and 200 nm are 2.44x10-14 m2 and 16.8x10-14 m2, respectively. In the same manner, resonantly calculated local field intensities displayed in Figure 4 (b) & (c) reveal that size of the Nano ring has paramount effect on the local field distribution.
Refractive index sensitivity of Au Nanodisc particle (D=200nm)
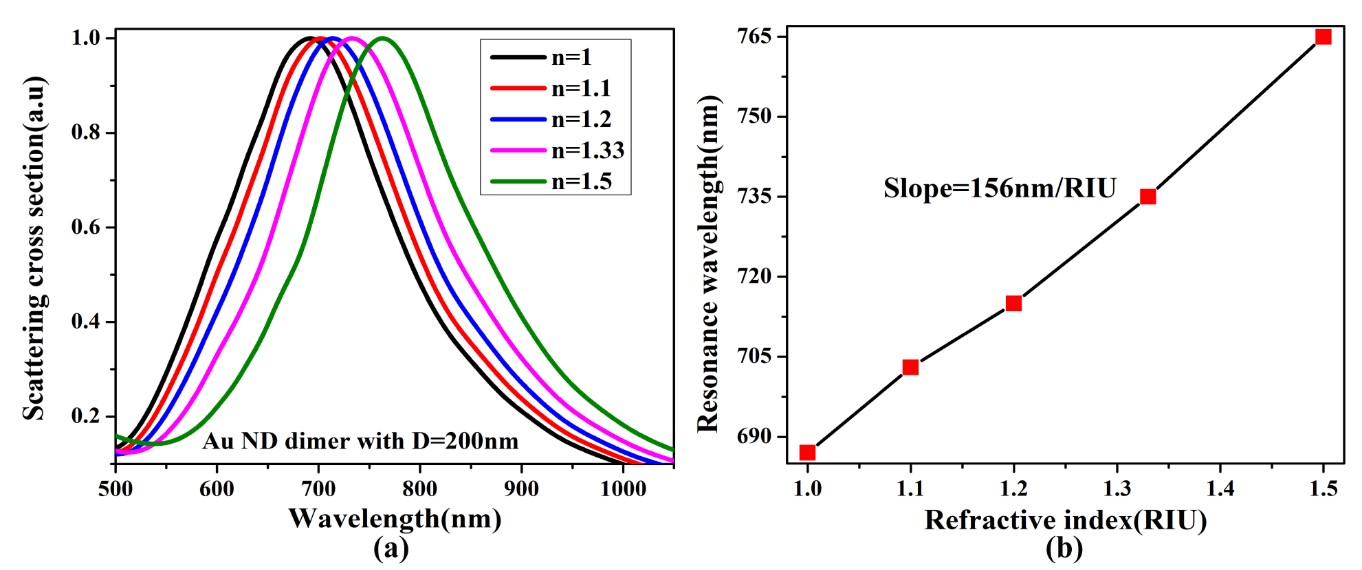
Figure 5(a) shows scattering spectra of a single Au ND with D=200nm as a function of a refractive index. It shows different resonance wavelengths for different refractive indices varying n=1 to 1.5. It is clear that while the refractive index of the ND increases from 1 to 1.5 the wavelength shifts from 687nm to 765nm. This was investigated by changing the simulation fixed values from 1 to 1.5 using FDTD. Fig 5(b) shows the slope of refractive indices of the ND against resonance wavelength. Hence, calculating its slope gives the sensitivity of Au ND with D=200nm is 156nm/RIU.
The above figure shows different resonance wavelengths with different refractive indices. As the refractive index increases from 1 to 1.5 the resonance wavelength shifts from 687 nm to 765 nm. Therefore, the sensitivity of Au ND dimer with D=200 nm is 156 nm/RIU.
Refractive index sensitivity of Au Nanoring with D=200nm
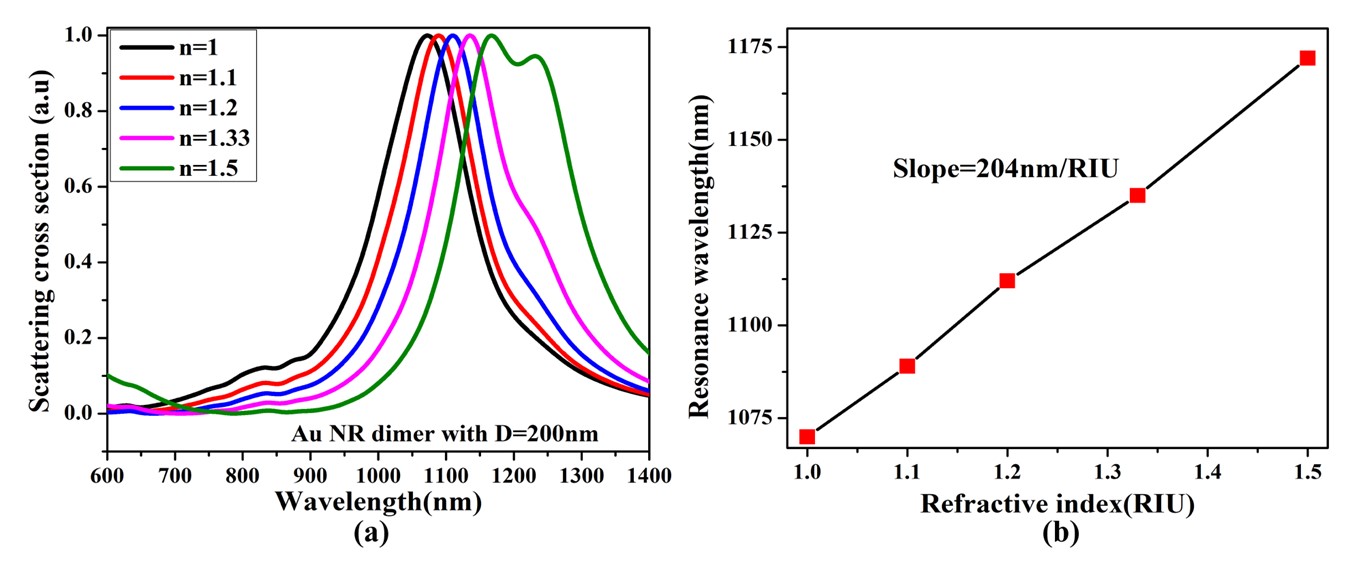
As displayed in Figure 6(a) and (b) the resonance wavelength of the Au Nanoring tends to shift as the refractive index of its surrounding changes. As the refractive index of the medium increases from 1 to 1.5 the resonance wavelength shifts from 1070nm to 1172nm. Fig 6(b) shows that the result of Au NR for different refractive indices while calculating the slope of the refractive index against resonance wavelength got the sensitivity of Au NR dimer with dimer D=200nm is 204nm/RIU. Therefore, it shows that for a single Au NR changing the refractive index enhanced its sensitivity.
This result, therefore, is often a way for adjusting the size, the surrounding medium and plasmonic wavelength of Au NPs enhanced the resonance wavelength shift; this increased the sensitivity of AU NR and Au ND respectively.
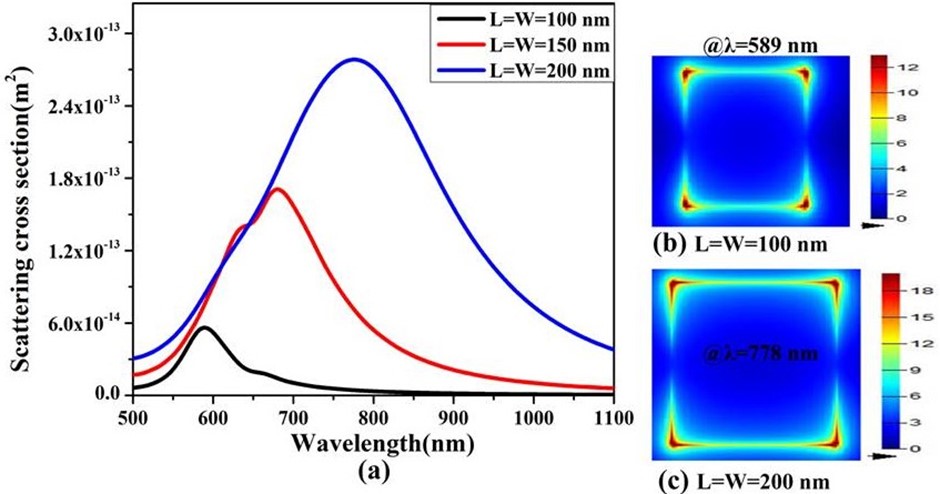
As displayed in figure 7 (a), the resonance wavelengths of Au Nano cube (NC) with length (L) = 100 nm and 200 nm are 589 nm and 778 nm, respectively. The resonance shift (Δλ) is 189 nm. The scattering cross section of Au NC with L=100 nm and 200 nm are 5.68x10-14 m2 and 27.9x10-14 m2, respectively. As shown in Figure 7(b) & (c), the local field intensities calculated resonant wavelengths for two different cube sizes show notable difference.
Conclusions
This paper was intended to design a highly sensitive nanoplasmonic biosensor based on novel AU nanoring and Nanodisc metallic structures with adjustable thickness, size, and dielectric environment. The design was based on the stepwise change of Au NR and ND with controllable thickness and Nano frameworks. The dipolar Plasmon resonance of Au nanoparticles can be varied from visible to near-infrared regions and by varying size of the Au nanoparticles (NPs) and changing refractive index of the surrounding medium. Note that this systematically designed nanosensor showed essential consideration to be used in future in investigating in LOC platforms.
Additionally, when the size of Au Nano disk, Nano cube and Nano ring increase, both far- and near-field properties of the nanoparticles show significant change. As the size of the disk, cube and ring nanoparticle increases, the scattering intensity increases whereas the spectral position of the surface Plasmon resonance tends to redshift according the FDTD simulation results.
Resonance shift properties of the gold are size and shape dependent. We can observe the effect of size and shape on Optical properties of metal nanoparticles, mostly gold nanoparticles, because they have a surface resonance in the visible region of the spectrum, as the Optical properties of metallic nanoparticles can be tuned to different wavelengths by changing their size and shape. It is clearly evident from the calculated spectra that the optical properties of nanoparticles are highly dependent on the nanoparticle size and shape. We can conclude that the resonance shift is strongly affected by the particle size and shape.
This study and results offer a new insight regarding designing biosensors based on plasmonic nanoparticles that are highly sensitive. Additionally, checking the performance of this theoretically designed nanosensor in biological platform will answer the limitation this work on application. It will provide many opportunities for developing Plasmon-enabled applications in biomedicine.
- Hill RT (2015) Plasmonic biosensors. WIREs Nanomed Nano-biotechnology 7: 152-68.
- Homola J, Yee SS, Gauglitz G (1999) Surface plasmon resonance sensors: a review. Sens Actuators B Chem 54: 3-15.
- Snook B (2012) Theory and practical application of surface plasmon resonance for analytical purposes. Theor Exp Chem 48: 283-306.
- Brolo AG (2012) Plasmonics for future biosensors. Nat Photonics 6: 709-13.
- Campbell CT, Kim G (2007) SPR microscopy and its applications to high-throughput analyses of bio molecular binding events and their kinetics. Biomaterials 28: 2380-92.
- Nelson BP, Grimsrud TE, Liles MR, Goodman RM, Corn RM (2001) Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption on to DNA micro arrays. Anal Chem 73: 1-7.
- Smith EA, Thomas WD, Kiessling LL, Corn RM (2003) Surface plasmon resonance imaging studies of protein-carbohydrate interactions J am Chem Soc 125: 6140-8.
- Mock JJ, Barbic M, Smith DR, Schultz DA, Schultz S (2002) Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys 116: 6755.
- Anker J, Hall W, Lyandres O, Shah N, Zhao J, VanDuyne RP (2008) Biosensing with plasmonic nano sensors.NatMater 7: 442-53.
- Dahlin AB, Wittenberg NJ, Höök FOhS-H (2013) Promises and challenges of nanoplasmonic devices for re fracto metric bio sensing. Nano photonics 2: 1-19
- Ament I, Prasad J, Henkel A, Schmachtel S, Sön-nichsen C (2012) Single unlabeled protein detection on individual plasmonic nanoparticles. NanoLett 1092-5.
- Chen SS vedendah lM, VanDuyne RP, Käll M (2011) Plasmon-enhanced colorimetric ELISA with single-molecule sensitivity. NanoLett 11: 1826-30.
- Nusz GJ, Marinakos SM, Rangarajan S, Chilkoti A (2011) Dual-order snapshot spectral imaging of plasmonic nanoparticles. Appl Opt 50: 4198-206.
- Guo L, Ferhan AR, Lee K, Kim DH (2011) Nanoarray-based biomolecular detection using individual Au nanoparticles with minimized localized surface plasmon resonance variations.AnalChem 83: 2605-12.
- http://www.marketsandmarkets.com/Market-Reports/biosen-sors-market-798.
- Homola J (2008) Surface plasmon resonance sensors for detection of chemical and biological species. Chem Rev 108: 462-93.
- ake O, Inci F, Demirci U (2014) Advances in plasmonic technologies for point of care applications. Chem Rev 114: 5728-52. T
- Gao Y, Gan Q, Bartoli FJ (2014) Break throughs in photonics 2013: research highlights biosensors based on plasmonic nanostructures. IEEE Photonics J 6: 1-5.
- Mayer KM, Hafner JH (2011) Localized surface plasmon resonance sensors.Chem Rev 111: 3828-57.
- Dahlin Andreas B, Wittenberg Nathan J, Höök F, OhS-H (2013) Promises and challenges of nanoplasmonic devices for re fractometric bio-sensing. Nano photonics 2: 83.
- Piliarik M, Sipova H, Kvasnicka P, Galler N, Krenn JR, Homola J (2012) High-resolution biosensor based on localized surface plasmons. Opt Express 20: 672-80.
- Sipova H, Vrba D, Homola J (2012) Analytical value of detecting an individual molecular binding event: the case of the surface plasmon resonance biosensor. Anal Chem 84: 30-3.
- Svedendah lM, Chen S, Dmitriev A, Kall M. Refractometric sensing using propagating versus localized surface plasmons: a direct comparison.Nano Lett 9: 4428-33.
- Svedendah lM, Chen S, Dmitriev A, Kall M. Refractometric sensing using propagating versus localized surface plasmons: a direct comparison.Nano Lett 9: 4428-33.
- Estevez MC, OtteMA, Sepulveda B, Lechuga LM (2014) Trends and challenges of refractometric nanoplasmonic biosensors: a review.AnalChim Acta 806: 55-73.
- So DWC, Seshadri SR (1997) Metal-island-film polarizer. Journal of the Optical Society of America B 14: 2831
- Lal U, Link S, Halas NJ (2007) Nano-optics from sensing to waveguiding. Nature Photonics 1: 641.
- MS Sharrouf, Awad R, Marhaba S, Bakeer DE (2016) Structural, optical and room temperature magnetic study of Mn-doped ZnO nanoparticles. Nano 11: 1650042.
- MS Sharrouf, Awad R, Roumié M, Marhaba S (2015) Structural, optical and room temperature magnetic study of Mn2O3 nanoparticles. Materials Sciences and Applications 5: 850.
- HR Stuart, Hall DG (1998) Island size effects in nanoparticle-enhanced photodetector. Applied Physics Letters 73: 3815.
- Akella A, Honda T, Liu AY, Hesselink L (1997) Two photon holographic recording in aluminok2silicate glass conta.ning silver particles. Optics Letters 22: 967.
- Ricard D, Roussignol P, Flytzanis C. Surface-mediated enhancement of optical phase
- U Kreibig, M Vollmer (1995) Optical Properties of Metal Clusters, Springer., Berlin.
- G Schmid (1994) Clusters and Colloids-From Theory to Applications, VCH. Weinheim, Germany.
- JA Creighton, CG Blatchford, MG Albrecht (1979) J Chem Soc. Faraday Trans. 75: 790.
- KU Von Raben, RK Chang BL. Laube Chem. Phys. Lett 79: 465.
- AA Lazarides, GC Schatz (2000) J Chem. Phys 112: 2982.
- K Drukker, G Wu, GC Schatz (2001) J Chem. Phys 114: 579.
- J. Chem. Educ 2010 7: 742-6
- Sensors (Basel) (2015) 15: 10481-510
- RIVM report 080116001(2013) Sensors 2022,22,1620 Micro fluidic POC Devices in Early Diagnosis
- Nanoscale Adv (2019) 1: 459-69
- Nanotechnology Reviews 2: 269-88.
- Biotechnology 44: 83-91
- NANOCS.net, 800-388, 2018
- Journal of controlled Release volume 353, January 2023, pages 699-712
- Malvern Panalytical LTD 2023
- Catalysts 2022, 12: 1386
- IJCSE’ERD ISSN 2249-6866, 3: 63-72

Figures at a glance