Docetaxel in Combination with Androgen Deprivation Therapy for Metastatic Hormone-Sensitive Prostate Cancer - A Single-Center Retrospective Study in Japan
Received Date:February 12, 2023 Accepted Date: March 03, 2023 Published Date:March 06, 2023
doi: 10.17303/jocr.2023.4.102
Citation: Toshihiro Saito, Kazuhiro Kobayashi, Takashi Kawasaki, Yasuo Kitamura, Toshiki Tanikawa (2023) Docetaxel in Combination with Androgen Deprivation Therapy for Metastatic Hormone-Sensitive Prostate Cancer - A Single-Center Retrospective Study in Japan.JJ Oncol Clin Res 4:1-14
Abstract
We characterized the effects of docetaxel (DTX) therapy for patients with metastatic hormone-sensitive prostate cancer (mHSPC) at our institution. We retrospectively analyzed 348 patients with newly diagnosed metastatic prostate cancer treated with androgen deprivation therapy (ADT) between 2006 and 2018. In total, 313 patients were treated with ADT alone (control group). The remaining 35 patients received ADT plus five DTX (60 mg/m2) cycles every 4 weeks without steroids (DTX group). This group had significantly better prostate-specific antigen-progression-free survival (PSA-PFS) and overall survival (OS) rates than the control group (Hazard ratio (HR) = 0.398, 95% confidence interval (CI) = 0.258–0.613, p < 00001, HR = 0.442, 95% CI = 0.256–0.761, p = 0.0032, respectively), although treatments were not randomized. In multivariate Cox proportional hazard analyses, the DTX group was still significantly associated with longer PSA-PFS and OS rates (HR = 0.402, 95% CI = 0.258–0.625, p < 0.0001, and HR = 0.526, 95% CI = 0.302–0.916, p = 0.0233, respectively). Thus, DTX therapy is an effective treatment for mHSPC patients in Japan, like other countries.
Keywords: Prostate Cancer; Docetaxel Therapy; mHSPC; Japan
Introduction
Since the 1940s, androgen deprivation therapy (ADT) has been the standard care for patients with metastatic hormone-sensitive prostate cancer (mHSPC) [1]. However, in the modern era, many life-extending therapies have been developed in combination with ADT [2], e.g., docetaxel (DTX), abiraterone acetate, enzalutamide, and apalutamide [3-7].
Of these, DTX was the first to improve the overall survival (OS) of men with mHSPC [8]. Three pivotal randomized phase III trials (GETUG15, CHAARTED, and STAMPEDE) demonstrated progression-free survival (PFS) benefits with DTX [8-12]. While the GETUG15 study demonstrated no major OS benefit, the other two studies did.
Given these results, DTX chemotherapy combined with ADT for mHSPC is now indicated in European and American guidelines [13]. However, Japanese guideline 2016 edition simply state, “In large-scale clinical trials overseas, it was reported that the prognosis was improved by using DTX chemotherapy in combination with first-line hormone therapy for metastatic prostate cancer [14].”
Recently, benefit of docetaxel chemotherapy for mHSPC began to be reported also in Japan [15,16]. To clarify the effect of docetaxel chemotherapy for Japanese patients, we conducted this retrospective study examined cases during the period when androgen-receptor-axis-targeted agents (ARAT), i.e., abiraterone, enzalutamide, or apalutamide, were not used in our institution.
Materials and Methods
Patients
We retrospectively analyzed clinicopathological and prognostic data from 348 patients with newly diagnosed metastatic prostate cancer treated with ADT at our institution between 2006 and 2018.
The study was approved by the institutional internal review board of Niigata Cancer Center Hospital (Niigata, Japan). The indication of DTX chemotherapy was determined by attending physicians, and treatment was performed for patients who provided written informed consent. All procedures conformed to the provisions of the Declaration of Helsinki.
The study was approved by the institutional internal review board of Niigata Cancer Center Hospital (Niigata, Japan). The indication of DTX chemotherapy was determined by attending physicians, and treatment was performed for patients who provided written informed consent. All procedures conformed to the provisions of the Declaration of Helsinki.
Of the 348 patients, 35 received DTX chemotherapy in combination with ADT as a first-line therapy (DTX group) and the remaining 313 (control group) received ADT alone.
All patients were pathologically diagnosed using ultrasound-guided prostate biopsy or transurethral re- section. The disease stage was determined using digital rectal examination (DRE), abdominal pelvic computed tomography (CT), bone scans, and thoracic CT or chest roentgenography in accordance with the 8th edition tumor-node-metastasis classification of the Union for Inter- national Cancer Control and the American Joint Committee on Cancer [17].
Patients were also separated into a low metastatic burden (extra regional lymph node metastasis or < four bone metastases without visceral metastasis) group and a high metastatic burden ( ≥four bone metastases or visceral metastasis) group following the definition in the CHAARTED Trial [10].
Treatments
DTX group received five DTX (60 mg/m2) cycles every 4 weeks in combination with ADT. We did not use prednisone in DTX group, since we have concerns about the sequelae of chronic steroid use, such as glucose intolerance, osteopenia, fluid retention and peptic ulcers, among other risks. The control group received ADT alone. ADT included an LH-RH agonist (Leuprorelin or Goserelin) or surgical castration plus anti-androgen therapy (Bicalutamide 80mg per day).
No patients received ARAT as a first-line treatment during the study.
Adverse events were graded using the Common Terminology Criteria on Adverse Events version 5.0 of the National Cancer Institute [18].
Statistical analyses
Categorical variable data were compared between groups using the Fisher’s exact test. Unpaired parameters were compared between groups using the Mann-Whitney U-test.
We defined OS and prostate-specific antigen-progression-free survival (PSA-PFS) as the period from treatment commencement to all-cause death, and PSA progression or death, respectively. PSA progression was defined as the earliest date where increased PSA ≥25% and ≥2 ng/mL values were observed.
Survival curves were generated using the Kaplan-Meier method and Log-rank tests were used for intertreatment comparisons. A Cox proportional hazards model was used for univariate and multivariate analyses to identify mortality risk factors. For multivariate analyses, we selected variables with p-value < 0.05 in univariate analyses. All tests were two-sided, and p < 0.05 was considered statistically significant. All statistical analyses were con- ducted using the Statview 5.0 software program (Abacus Concepts, Berkley, CA, USA).
Results
Baseline patient characteristics are shown (Table 1). The age at diagnosis was significantly lower in the DTX group than the control group (p < 0.0001).
Moreover, significantly more patients in the control group had an Eastern Cooperative Oncology Group performance status (PS) ≥1.
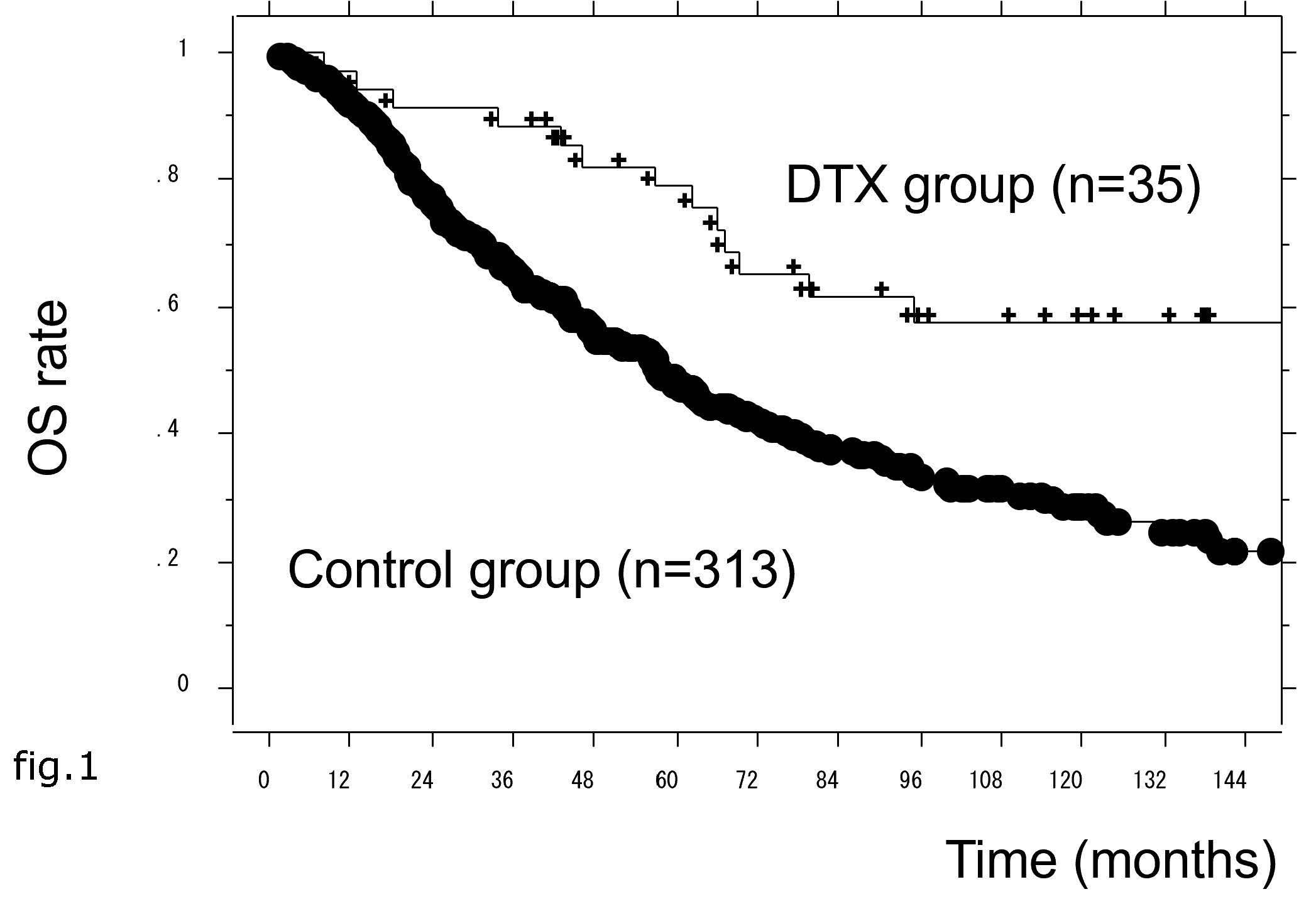
In total, 298 patients (85.6%) displayed PSA progression during primary treatment and the median PSA-PFS was 15 months. This period was significantly longer in the DTX group than the control group (Hazard Ratio (HR) = 0.398, 95% Confidence Interval (CI) = 0.258–0.613, p < 0.0001) (Figure 1).
In univariate Cox proportional hazard analyses, age <75 years, PS = 0, PSA <200 ng/mL, low metastatic burden, Gleason score (GS) <9, and DTX therapy were significantly associated with longer PSA-PFS periods. Moreover, in multivariate analyses, DTX therapy remained significantly associated with longer PSA-PFS periods (HR = 0.402, 95% CI = 0.25–0.625, p < 0.0001) (Table 2).
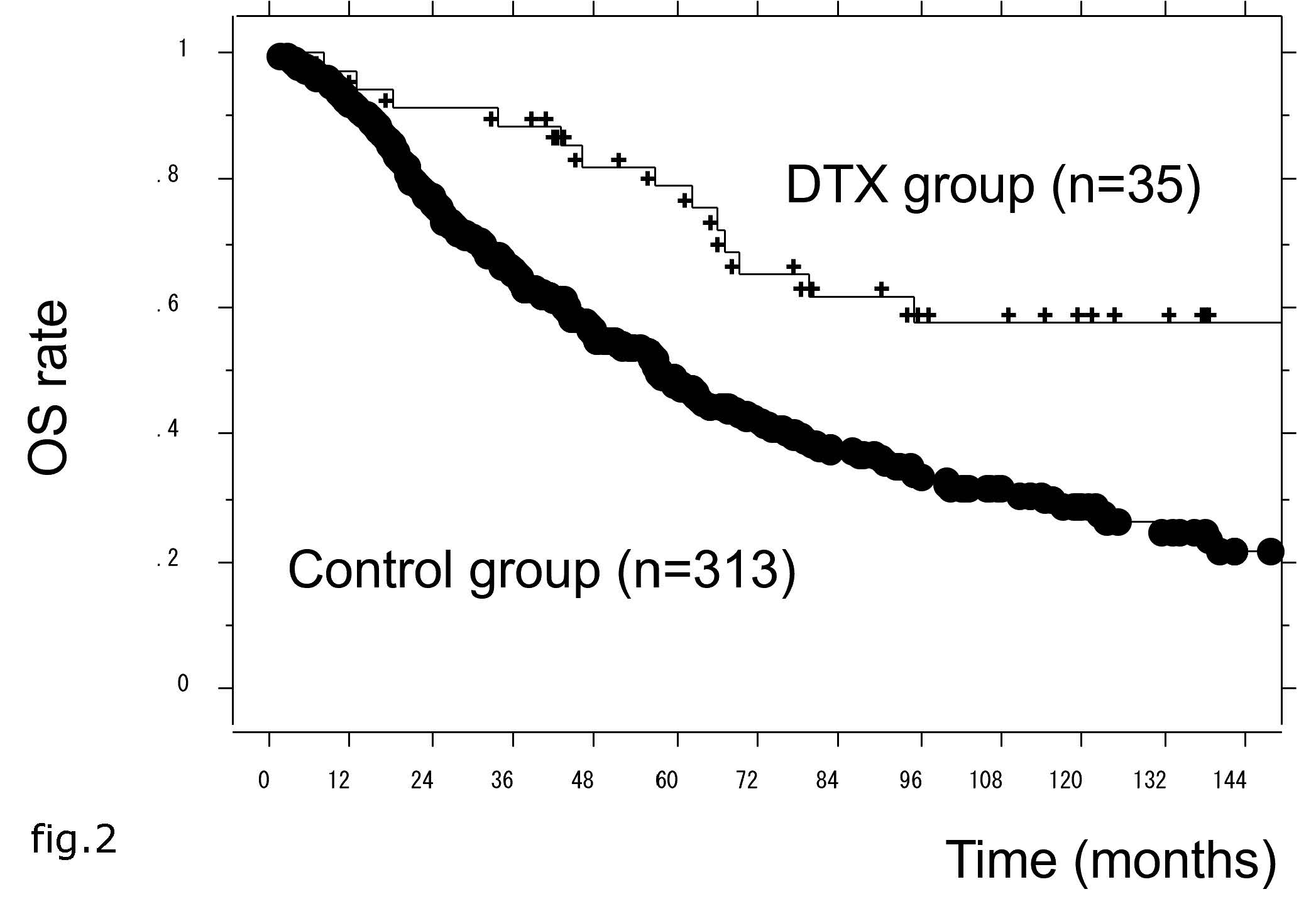
The median OS of all patients was 62 months, but this was significantly longer in the DTX group than the control group (HR = 0.442, 95% CI = 0.256–0.761, p = 0.0032) (Figure 2).
In univariate Cox proportional hazard analyses, age <75 years, PS = 0, low metastatic burden, GS <9, and DTX therapy were significantly associated with longer OS. In multivariate analysis, DTX therapy remained significantly associated with longer OS (HR = 0.526, 95% CI = 0.302–0.916, p = 0.0233) (Table 3).
Moreover, in subgroup analyses, PSA-PFS and OS were analyzed in high and low metastatic burden cohorts, respectively. PSA-PFS was significantly longer in the DTX group than the control group, as observed in both the high and low metastatic burden cohorts (Figures 3a and 3b).
In multivariate analysis, DTX therapy remained significantly associated with longer PSA-PFS periods (Tables 4a and 4b).
However, OS was significantly longer in the DTX group than the control group, as observed only in the high metastatic burden cohort (Figures 4a and 4b).
Moreover, in multivariate analysis, DTX therapy was not significantly associated with longer OS times, even in the high metastatic burden cohort (Tables 5a and 5b).
In the DTX group, the most common toxicity issue was neutropenia; grade 3 or 4 was observed in 16 patients and febrile neutropenia in one, but no grade 5 toxicity was observed. Adverse effects requiring dis- continuation of docetaxel were observed in two patients. One discontinued after only one course of docetaxel due to bloody stools, and another patient after two courses due to arthritis.
Discussion
DTX is a first-line chemotherapy agent for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC), and was the first drug to demonstrate improved OS for mCRPC [19,20]. Following the establishment of DTX therapy for mCRPC, the pertinent question was whether to administer che- motherapy to patients who were sensitive to hormone therapy to improve patient outcomes.
Since prostate cancer cell growth is driven by androgens, ADT is the standard treatment for hormone naïve disease. However, despite reliable initial responses, disease progression is ultimately inevitable. The hypothesis that a subpopulation of prostate cancer cells may be hormone-resistant, and thus resistant to ADT from the beginning, formulated a rationale to combine ADT with chemotherapy in men with hormone-sensitive disease [21].
Indeed, many investigators have considered the early application of chemotherapeutic agents in combination with ADT. Three randomized clinical trials conducted in the 1980s examined the role of early combined chemotherapy plus hormone therapy to treat mHSPC [22-24]. However, these studies did not demonstrate a survival benefit for patients on chemotherapy plus ADT. While these early cytotoxic therapy trials were continued with some alterations, a clear clinical benefit was not identified [25,26].
Previously, we reported promising results of chemo-endocrine therapy using a VIP (Vincristine, Ifosfamide, Pepleomycin) regimen [27], however, it was a non-randomized, retrospective study, and the conclusions were not definitive.
However, at that era, the clinical benefit of chemotherapy had not been established, even for mCRPC. Since then, DTX therapy has improved mCRPC patient survival [19,20], thus raising the possibility of early use with chemotherapy for mHSPC once more.
In this study, we began chemo-endocrine therapy using DTX for mHSPC with careful patient selection and informed consent based on our former experience with chemo-endocrine therapy using VIP regimen [27]. With respect to patient safety, we used a low DTX dose and a longer interval for the drug course.
Although our protocol included a low DTX dose and had one fewer course than the phase III clinical trials, the prognoses in patients receiving DTX chemotherapy were better than those receiving ADT alone. Since there was a significant difference in age and PS in the patient's background, we also performed a multivariate analysis. The results also show that this treatment improved survival.
A few studies have been reported on the results of docetaxel treatment for Japanese mHSPC patients [15,16], and the significance of this treatment has not been sufficiently verified because ARAT is currently often used for mHSPC in Japan,
Our results suggested that data from randomized phase III trials in other countries could also be applied to Japanese settings, indicating this treatment could be used as a standard therapy for mHSPC in Japan.
However, in these trials, high metastatic burden cases primarily benefited from this treatment [28]. Therefore, we also performed subgroup analyses in high and low metastatic burden groups.
Although in the low metastatic burden group, DTX therapy failed to show significant survival gain, in the high metastatic burden group, DTX showed significant survival benefits. These observations suggested that DTX therapy was more effective in high metastatic burden groups than low burden group, in agreement with these trials [10,11].
However, in our subgroup analyses, we failed to observe significant results in multivariate analyses, probably because case numbers were small. However, we believe this could be clarified by increasing case numbers in future studies.
Recently, ARAT was clinically used as an initial treatment for mHSPC and has rapidly spread across Japan. In June 2018, our institution commenced ARAT for mHSPC, and this has been increasing. To comprehensively clarify the effects of upfront DTX chemotherapy, we examined cases when ARAT was not used in our institution. Therefore, no patients receiving ARAT as an initial treatment were included in the control group in this study. However, whether chemotherapy or ARAT should be used as an initial treatment for mHSPC remains a perplexing issue.
A comparison of treatments used in this study were reported in a meta-analysis [29], but more data from randomized studies are required. In addition, combination therapy studies have begun elsewhere [30,31].
Currently, treatment options for mHSPC patients are increasing and patient benefits are emerging, however, there is no firm policy on drug selection so far.
Inevitably, our study included some bias because it was a retrospective, non-randomized study with relatively small cases at a single institution. Thus, a well-designed trial with more statistical power is required to confirm this approach is beneficial for newly diagnosed Japanese patients with mHSPC. However, we were able to show the local situation of prostate cancer treatment in Japan, which is different from Western countries.
Conclusion
Our results suggest that DTX chemotherapy plus ADT improves the survival of Japanese patients with mHSPC. Further studies are warranted to confirm the efficacy and safety of this approach.
Acknowledgments
The authors thank Drs. S. Komatsubara, S. Wakatsuki, S. Hoshino, T. Nobushita, H. Yamazaki, V. Bilim, T. Toba, K. Takeda, S. Ishikawa, A. Kazama, S. Yamaguchi, K. Inui, M. Murata, E. Yuki, M. Hasegawa, and K. Watanabe for their valuable contribution to patient treatment.
Disclosure
The author reports no conflicts of interest in this work.
- Huggins C, Hodges CV (1941) Studies on prostate cancer. Effect of castration, estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1: 293-7
- Shiota M, Eto M (2016) Current status of primary pharmacotherapy and future perspectives toward upfront therapy for metastatic hormone-sensitive prostate cancer. Int. J. Urol 23: 360-9.
- Sweeney CJ, Chen YH, Carducci M et al. (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med 373: 737-46.
- Fizazi K, Tran NP, Fein L et al. (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 2017; 377: 352–60. Clinical Status Trials. JAMA 297: 1332-43.
- Davis ID, Martin AJ, Stockler MR et al. (2019) Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl. J. Med 381: 121-31
- Armstrong AJ, Szmulewitz RZ, Petrylak DP et al. (2019) ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J. Clin. Oncol 37: 2974-86.
- Chi KN, Agarwal N, Bjartell A et al. (2019) Apalutamide for Metastatic, castration-sensitive prostate cancer. N. Engl. J. Med 381: 13-24.
- Gravis G, Fizazi K, Joly F et al. (2013) Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomized, open-label, phase 3 trial. Lancet Oncol 14: 149-58.
- Gravis G, Boher JM, Joly F et al. (2016) Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur. Urol 70: 256-62.
- Kyriakopoulos CE, Chen YH, Carducci MA et al. (2018) Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol 36: 1080-7.
- James ND, Sydes MR, Clarke NW et al. (2016) Adding docetaxel and/or zoledronic acid for hormone-naive prostate cancer (STAMPEDE): survival results form an adaptive multi-arm multi-stage platform randomized controlled trial. Lancet 387: 1163-77.
- Clarke NW, Ali A, Ingleby FC et al. (2019) Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann. Oncol 30: 1992-2003.
- Lowrance WT, Breau RH, Chou R et al. (2021) Advanced prostate cancer: AUA/ASTRO/SUO guideline part I. J. Urol 205: 14-21.
- Kakehi Y (2016) Clinical Practice Guideline for Prostate Cancer. Medical View. Tokyo.
- Muto Y, Narita S, Hatakeyama S et al. (2021) Short-term outcomes of risk-adapted upfront docetaxel administration in patients with metastatic hormone-sensitive prostate cancer: a multicenter prospective study in Japan. Med. Oncol 38: 37.
- Yanagisawa T, Kimura T, Hata K et al. (2022) Combination of docetaxel versus nonsteroidal antiandrogen with androgen deprivation therapy for high-volume metastatic hormone-sensitive prostate cancer: a propensity score-matched analysis. World J Urol.
- Brierley JD, Gospodarowicz MK, Wittekind C (2016) TNM classification of Malignant Tumours 8th edition. Wiley-Blackwell.
- Department of Health and Human Services US (2017) Common terminology criteria for adverse events (CTCAE) version 5. National Institutes of Health.
- Tannock IF, de Wit R, Berry WR et al. (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med 351: 1502-12.
- Petrylak DP, Tangen CM, Hussain MH et al. (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med 351: 1513-20.
- Isaacs JT, Coffey DS (1981) Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy studied in the Dunning R.−3327-H adenocarcinoma. Cancer Res 41: 5070-5.
- Murphy GP, Beckley S, Brady MF et al. (1983) Treatment of newly diagnosed metastatic prostate cancer patients with chemotherapy agents in combination with hormones versus hormones alone. Cancer 51: 1264-72.
- Murphy GP, Huben RP, Priore R (1986) Results of another trial of chemotherapy with and without hormones in patients with newly diagnosed metastatic prostate cancer. Urology 28: 36-40.
- Huben RP, Murphy GP (1988) A comparison of diethylstilbestrol or orchiectomy with buserelin and with methotrexate plus diethylstilbestrol or orchiectomy in newly diagnosed patients with clinical stage D2 cancer of the prostate. Cancer 62: 1881-7.
- Millikan RE, Wen S, Pagliaro LC et al. (2008) Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J. Clin. Oncol 26: 5936-42
- Noguchi M, Noda S, Yoshida M et al. (2004) Chemohormonal therapy as primary treatment for metastatic prostate cancer: A randomized study of estramustine phosphate plus luteinizing hormone-releasing hormone agonist versus flutamide plus luteinizing hormone-releasing hormone agonist. Int. J. Urol 11: 103-9.
- Saito T, Kitamura Y, Komatsubara S (2005) Chemo-endocrine therapy for newly diagnosed stage D2 prostate cancer. Hinyokika Kiyo 51: 789-92.
- Gravis G, Boher JM, Chen YH et al. (2018) Burden of metastatic castrate naive prostate cancer patients, to identify men more likely to benefit from early docetaxel: further analyses of CHAARTED and GETUG-AFU15 studies. Eur. Urol 73: 847-55.
- Mori K, Mostafaei H, Sari Motlagh RS et al. (2021) Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int.
- Fizazi K, Maldonado X, Foulon S et al. (2021) A phase 3 trial with a 2×2 factorial design of abiraterone acetate plus prednisone and/or local radiotherapy in men with de novo metastatic castration-sensitive prostate cancer (mCSPC): first results of PEACE-1. J. Clin. Oncol 39.
- Matthew RS, Maha H, Fred S et al. (2022) Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med 386: 1132-42.

.jpg)
.jpg)
.jpg)
.jpg)
Tables at a glance
Figures at a glance