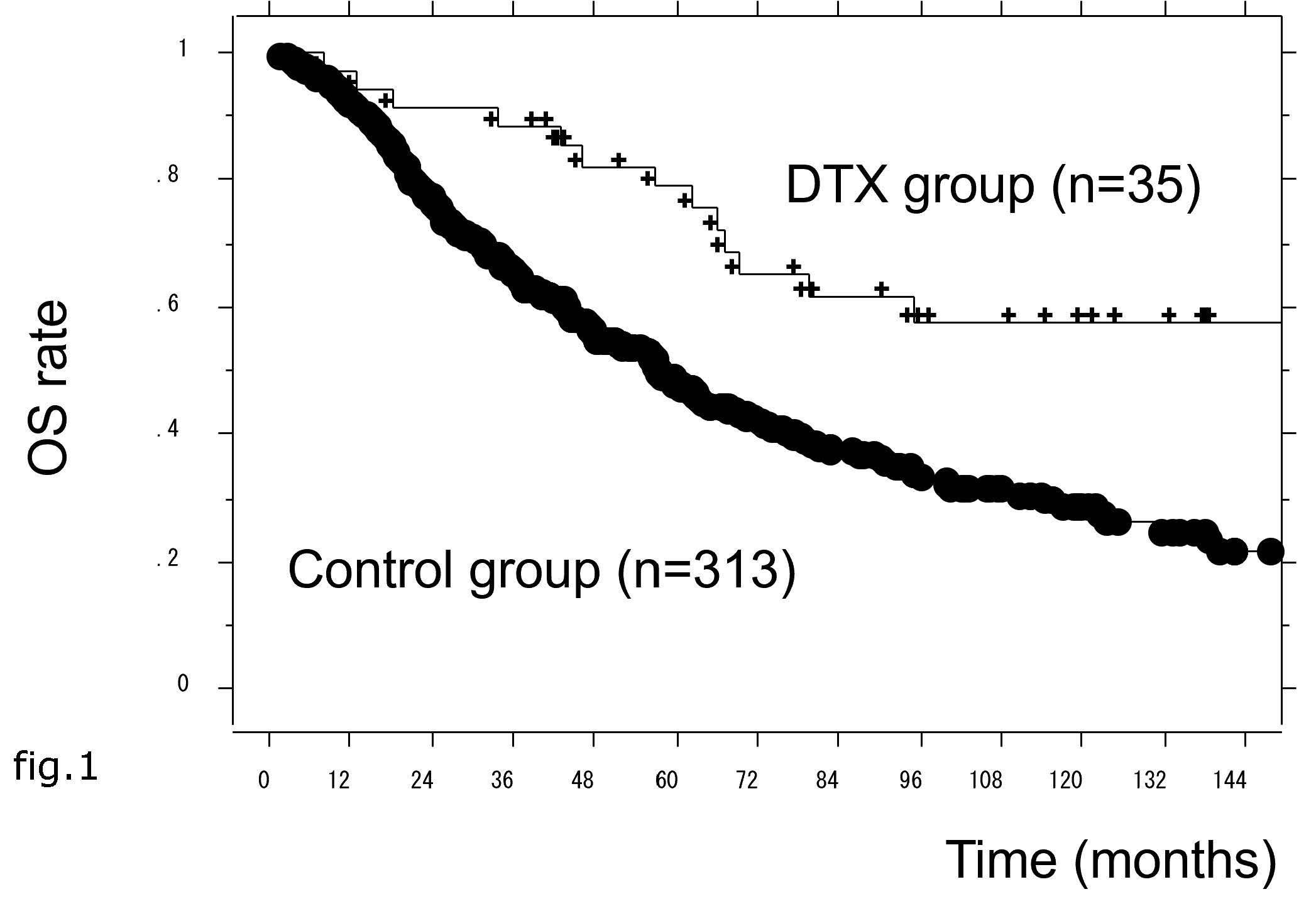
Figure 1: The prostate-specific antigen progression-free survival (PSA-PFS) in docetaxel (DTX) and control patient groups
DTX group |
Control group |
P-value |
|
Number of patients |
35 |
313 |
|
Months of follow-up, mean (range) |
82.1 (8–152) |
55.1 (1–180) |
|
Age, mean (range) |
66.0 (46–77) |
74.5 (47–101) |
P < 0.0001 |
PS |
|||
0 |
33 |
230 |
P = 0.0059 |
1 or greater |
2 |
83 |
|
PSA ng/ml, mean (range) |
480.6 (3.21–3 416) |
878.1 (1.24–19362) |
P = 0.2240 |
Gleason score |
|||
≤8 |
8 |
70 |
P = 0.9471 |
≥9 |
27 |
243 |
|
cT |
|||
≤T3a |
19 |
149 |
P = 0.5674 |
≥T3b |
16 |
164 |
|
cN |
|||
N0 |
12 |
119 |
P = 0.6655 |
N1 |
23 |
194 |
|
cM |
|||
M1a |
4 |
50 |
P = 0.6464 |
M1b |
25 |
223 |
|
M1c |
6 |
40 |
|
Metastatic burden |
|||
Low |
14 |
135 |
P = 0.8611 |
High |
21 |
178 |
PS, Eastern Cooperative Oncology Group Performance Status; cT, clinical T-stage; cN, clinical N-stage; cM, clinical M-stage; PSA, prostatespecific
antigen; DTX, docetaxel
Table 1: Baseline patient characteristics
|
Univariate analysis |
Multivariate analysis |
||||
|
Odds |
95% CI |
P-value |
Odds |
95% CI |
P-value |
Age (<75/≥75) |
0.795 |
0.633–0.998 |
0.0481 |
0.918 |
0.726–1.161 |
0.4763 |
PS (0/≥1) |
0.719 |
0.551–0.937 |
0.0147 |
0.869 |
0.661–1.141 |
0.3124 |
PSA (<200/≥200) |
0.655 |
0.520–0.824 |
0.0003 |
0.793 |
0.623–1.011 |
0.0607 |
cT (≤T3a/≥T3b) |
0.804 |
0.639–1.013 |
0.0639 |
|||
cN (N0/N1) |
0.875 |
0.691–1.108 |
0.2668 |
|||
Met. burden (low/high) |
0.569 |
0.450–0.718 |
<0.0001 |
0.570 |
0.448–0.726 |
<0.0001 |
GS (≤8/≥9) |
0.762 |
0.578–1.006 |
0.0549 |
|||
DTX (y/n) |
0.398 |
0.258–0.613 |
<0.0001 |
0.402 |
0.258–0.625 |
<0.0001 |
PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; cT, clinical T-stage; cN, clinical N-stage; Met,
Metastatic; GS, Gleason score; DTX, docetaxel
Table 2: Univariate and multivariate association analyses between different parameters and prostate-specific antigen-progression-free
survival
|
Univariate analysis |
Multivariate analysis |
||||
|
Odds |
95% CI |
P-value |
Odds |
95% CI |
P-value |
Age (<75/≥75) |
0.513 |
0.388–0.676 |
<0.0001 |
0.556 |
0.417–0.741 |
<0.0001 |
PS (0/≥1) |
0.541 |
0.400–0.731 |
<0.0001 |
0.688 |
0.503–0.940 |
0.0188 |
PSA (< 200/≥200) |
0.839 |
0.638–1.104 |
0.2102 |
|||
cT (≤T3a/≥T3b) |
0.838 |
0.636–1.105 |
0.2097 |
|||
cN (N0/N1) |
0.839 |
0.630–1.117 |
0.2289 |
|||
Met. burden (low/high) |
0.711 |
0.538–0.941 |
0.0170 |
0.698 |
0.526–0.927 |
0.0128 |
GS (≤8/≥9) |
0.679 |
0.482–0.955 |
0.0260 |
0.660 |
0.468–0.930 |
0.0174 |
DTX (y/n) |
0.442 |
0.256–0.761 |
0.0032 |
0.526 |
0.302–0.916 |
0.0233 |
PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; cT, clinical T-stage; cN, clinical N-stage; Met,
Metastatic; GS, Gleason score; DTX, docetaxel
Table 3: Univariate and multivariate association analyses between different parameters and overall survival
|
Univariate analysis |
Multivariate analysis |
||||
|
Odds |
95% CI |
P-value |
Odds |
95% CI |
P-value |
Age (<75/≥75) |
0.837 |
0.584–1.200 |
0.3326 |
|||
PS (0/≥1) |
0.710 |
0.455–1.109 |
0.1321 |
|||
PSA (<200/≥200) |
0.907 |
0.626–1.316 |
0.6077 |
|||
cT (≤T3a/≥T3b) |
0.647 |
0.447–0.938 |
0.0216 |
0.775 |
0.528–1.137 |
0.1922 |
cN (N0/N1) |
0.803 |
0.546–1.180 |
0.2637 |
|||
GS (≤8/≥9) |
0.631 |
0.404–0.986 |
0.0432 |
0.670 |
0.421–1.066 |
0.0910 |
DTX (y/n) |
0.346 |
0.166–0.723 |
0.0048 |
0.366 |
0.173–0.776 |
0.0087 |
PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; cT, clinical T-stage; cN, clinical N-stage; GS,
Gleason score; DTX, docetaxel.
Table 4(a): Univariate and multivariate association analyses between different parameters and prostate-specific antigen progression-free
survival in the low metastatic burden cohort
|
Univariate analysis |
Multivariate analysis |
||||
|
Odds |
95% CI |
P-value |
Odds |
95% CI |
P-value |
Age (<75/≥75) |
0.755 |
0.561–1.015 |
0.0630 |
|||
PS (0/≥1) |
0.792 |
0.569–1.103 |
0.1680 |
|||
PSA (<200/≥200) |
0.642 |
0.468–0.880 |
0.0059 |
0.702 |
0.511–0.964 |
0.0289 |
cT (≤T3a/≥T3b) |
0.989 |
0.736–1.329 |
0.9406 |
|||
cN (N0/N1) |
0.850 |
0.629–1.147 |
0.2873 |
|||
GS (≤8/ 9) |
0.871 |
0.612–1.239 |
0.4412 |
|||
DTX (y/n) |
0.396 |
0.232–0.677 |
0.0007 |
0.429 |
0.250–0.736 |
0.0021 |
PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; cT, clinical T-stage; cN, clinical N-stage; GS,
Gleason score; DTX, docetaxel
Table 4(b): Univariate and multivariate association analyses between different parameters and prostate-specific antigen progression-free
survival in the high metastatic burden cohort
|
Univariate analysis |
Multivariate analysis |
||||
|
Odds |
95% CI |
P-value |
Odds |
95% CI |
P-value |
Age (<75/≥75) |
0.427 |
0.272–0.670 |
0.0002 |
0.492 |
0.302–0.770 |
0.0022 |
PS (0/≥1) |
0.434 |
0.263–0.715 |
0.0011 |
0.546 |
0.325–0.918 |
0.0224 |
PSA (<200/≥200) |
0.953 |
0.604–1.502 |
0.8346 |
|||
cT (≤T3a/≥T3b) |
0.378 |
0.436–1.056 |
0.0856 |
|||
cN (N0/N1) |
0.786 |
0.488–1.266 |
0.3224 |
|||
GS (≤8/≥9) |
0.618 |
0.355–1.075 |
0.0886 |
|||
DTX (y/n) |
0.367 |
0.134–1.004 |
0.0509 |
|||
PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; cT, clinical T-stage; cN, clinical N-stage; GS,
Gleason score; DTX, docetaxel
Table 5(a): Univariate and multivariate association analyses between different parameters and overall survival in the low metastatic burden
cohort
|
Univariate analysis |
Multivariate analysis |
||||
|
Odds |
95% CI |
P-value |
Odds |
95% CI |
P-value |
Age (<75/≥75) |
0.572 |
0.402–0.813 |
0.0019 |
0.613 |
0.428–0.877 |
0.0074 |
PS (0/≥1) |
0.637 |
0.435–0.931 |
0.0200 |
0.740 |
0.502–1.091 |
0.1285 |
PSA (<200/≥200) |
0.882 |
0.608–1.281 |
0.5105 |
|||
cT (≤T3a/≥T3b) |
1.016 |
0.713-1.448 |
0.9308 |
|||
cN (N0/N1) |
0.829 |
0.577–1.192 |
0.3115 |
|||
GS (≤8/≥9) |
0.713 |
0.462–1.100 |
0.1258 |
|||
DTX (y/n) |
0.462 |
0.241–0.884 |
0.0198 |
0.523 |
0.270–1.012 |
0.0542 |
PS, Eastern Cooperative Oncology Group Performance Status; PSA, prostate-specific antigen; cT, clinical T-stage; cN, clinical N-stage; GS,
Gleason score; DTX, docetaxel
Table 5(b): Univariate and multivariate association analyses between different parameters and overall survival in the high metastatic burden
cohort

Figure 1: The prostate-specific antigen progression-free survival (PSA-PFS) in docetaxel (DTX) and control patient groups
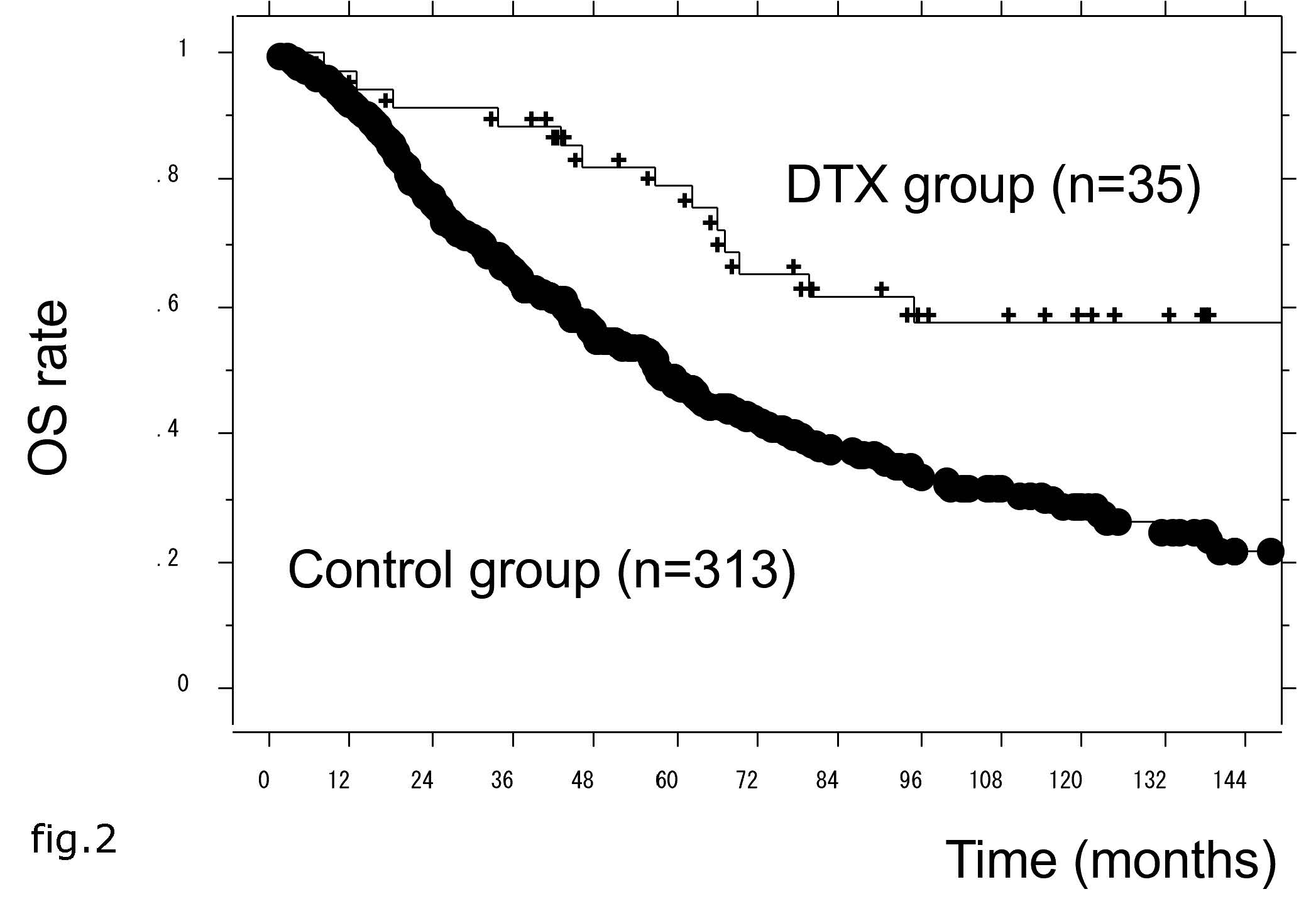
Figure 2: The overall survival (OS) rate in docetaxel (DTX) and control patient groups
.jpg)
Figure 3(a): The prostate-specific antigen progression-free survival (PSA-PFS) rate in docetaxel (DTX) and control patient groups in the low metastatic burden cohort
.jpg)
Figure 3(b): The overall survival (OS) rate in docetaxel (DTX) and control patient groups in the low metastatic burden cohort
.jpg)
Figure 4(a): The prostate-specific antigen progression-free survival (PSA-PFS) rate in docetaxel (DTX) and control patient groups in the high metastatic burden cohort
.jpg)
Figure 4(b): The overall survival (OS) rate in docetaxel (DTX) and control patient groups in the high metastatic burden cohort
Tables at a glance
Figures at a glance