Reticulocyte Hemoglobin Equivalent as an Early Predictor of Iron Deficiency Anemia in Cancer Patients
Received Date: April 25, 2023 Accepted Date: May 25, 2023 Published Date: May 27, 2023
doi: 10.17303/jocr.2023.4.103
Citation: Aditya Sarin, Amit Agarwal, Chandragouda Dodagoudar, Saphalta Baghmar, Suhail Qureshi (2023) Reticulocyte Hemoglobin Equivalent as an Early Predictor of Iron Deficiency Anemia in Cancer Patients.JJ Oncol Clin Res 4: 1-10
Abstract
Reticulocyte hemoglobin equivalent (RET-He) measures the amount of hemoglobin in the reticulocytes and indicates red cell hemoglobinization, reflecting the quality of the newly produced reticulocytes. It is a useful parameter which allows the diagnosis of IDA before it manifests clinically in a patient and also for treatment follow-up.
Therefore, the ability to assess iron deficiency, as part of automated CBC/reticulocyte analysis, may significantly enhance patient management. Etiology of CRA is multifactorial, and is due to myelosuppression secondary to chemotherapy, bone marrow failure, chronic inflammation, and/or iron deficiency. The goal of the present study was to establish a RET-He cut-off that could rapidly predict iron deficiency.
The data suggests that RET-He, at a threshold of 32 pg/ cell, may be an important discriminator to rule out IDA in patients with cancer.
Methods
Patients were enrolled into the study based on the laboratory test values of CBC and serum iron studies.
Results
The statistical analysis was performed using (A) biochemical standards for ID i.e. serum iron less than 40 µg/dl, transferrin saturation less than 20% as well as (B) us in house standards i.e. serum iron less than 40 µg/dl, transferrin saturation less than 30% with a RET-He cut-off at 30.4 pg. The PPV was calculated at 70.67 % and 75.9% for A & B respectively. The results were statistically significant in both (A) and (B) i.e. P value <0.001 as per table
Conclusion
RET-He is a readily available parameter on automated hematology analyzers with no additional cost and effort. Hence, we recommend the incorporation of RET-He in the diagnostic algorithm for the rapid assessment of IDA in the oncology practice as a surrogate marker with cut-off value <30.4 pg which had a PPV of 76%.This will negate the requirement of iron biochemistry studies in CRA patients, expedite the process of anemia correction with I.v. iron therapy and further reduce the requirement of additional blood sampling, along with providing cost efficacy to the patient especially in the cost-limited healthcare settings. In case of non-responders, a further extensive work up should be performed to delineate the etiology of CRA.
Keywords: Cancer Related Anemia; Ret-he; Iron transfusion; Supportive Care
Introduction
Reticulocyte hemoglobin equivalent (RET-He) measures the amount of hemoglobin in the reticulocytes and indicates red cell hemoglobinization, reflecting the quality of the newly produced reticulocytes [1]. It is also designated as cellular hemoglobin content of reticulocytes (CHr) on certain hematology analyser platforms. It is thought to reflect iron content in reticulocytes and is emerging as an alternative parameter that can be used as a tool for evaluation and sub-classification of different types of anemia, particularly iron deficiency anemia (IDA) [2]. Although, a standardized cut-off value of RET-He has not been established yet for cancer related anemia (CRA), numerous studies have already been reviewed for its cut-off values in specific patient populations such as pediatric, geriatric, pregnant, blood donors and hemodialysis patients etc. [3-11] It is a useful parameter which allows the diagnosis of IDA before it manifests clinically in a patient and also for treatment follow-up. Therefore, the ability to assess iron deficiency, as part of automated CBC/reticulocyte analysis, may significantly enhance patient management. Etiology of CRA is multifactorial, and is due to myelosuppression secondary to chemotherapy, bone marrow failure, chronic inflammation, and/or iron deficiency. The goal of the present study was to establish a RET-He cut-off that could rapidly predict iron deficiency. The data suggests that RET-He, at a threshold of 32 pg/ cell, may be an important discriminator to rule out IDA in patients with cancer.
Materials and Methods
A cross-sectional & observational study was conducted in the Department of Medical Oncology, BLK-Max Super Specialty Hospital, New Delhi over a period of 2 years (2018-2020) after obtaining clearance from ethical committee. The patients included in the study were above the age of 18 years of either sex with primary diagnosis of CRA after hematological evaluation, ECOG performance status 0-2, hemoglobin <10 gm/dl. Patients in the immediate post-operative period (3 weeks) and/or critically ill, diagnosed with leukemia, macrocytosis, thalassemia, recent history of Packed Red Blood Cells (PRBC) transfusion in past 3 weeks, taking erythropoietin stimulating agents (ESA), oral/intravenous iron preparations were excluded. Hematological investigations were performed on Mindray BC 6800 Plus Auto Hematology analyzer in the Department of Hematology. Iron studies were performed with Electrochemiluminescence (ECLIA) for S.Ferritin, Ferrozine for S. Iron and Pyridyl azo dye for Total Iron Binding Capacity (TIBC) estimation. The gold standard for assessment of iron stores is bone marrow examination. This is not performed in our hospital for sole purpose to identify iron deficiency due to its invasive nature. Apart from a complete hemogram, an iron profile including serum ferritin, serum iron, TIBC and Transferrin saturation (TSAT), are the most common biochemical tests used for the diagnosis of iron deficiency (ID) which is seen in both IDA and anemia of chronic disease (ACD). In the present study, IDA was defined biochemically as Hb <10gm/dl, serum iron <40 mcg/dl, transferrin saturation <20%, with or without ferritin <100ng/ml consistent with current clinical recommendations.
Statistical Analysis
Data was analyzed by using statistical software SPSS version 22. Qualitative variables were represented in form of frequency and percentage. Quantitative data was represented in form of mean and standard deviation. Receiver Operating Characteristic (ROC) curve was plotted to calculate threshold/cut-off values for RET-He. Sensitivity, specificity, and positive and negative predictive values were calculated for RET-He against routinely ordered serum iron studies as the diagnostic standard. Statistical differences between data sets were defined by a p value < 0.05.
Results
Patient and characteristics
ID was seen higher in females (n=161) with a younger age of incidence (54 ± 12.4 years) as compared to males (n=88, 61.8± 10.3 years). Rest all parameters were comparable as shown in table 1.
Distribution of RET-He
Patient and characteristics
ID was seen higher in females (n=161) with a younger age of incidence (54 ± 12.4 years) as compared to males (n=88, 61.8± 10.3 years). Rest all parameters were comparable as shown in table 1.
Distribution of RET-He
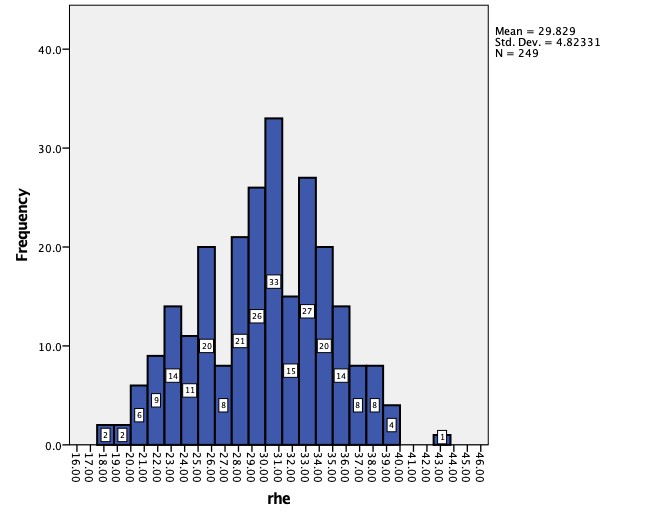
The distribution of RET-He values (pg) in the study population is illustrated in Figure No. 1. Values ranged from 17.6 to 43.0 pg. RET-He values ranging from 28 to 36 pg are generally considered normal.
ROC Curve for RET-He
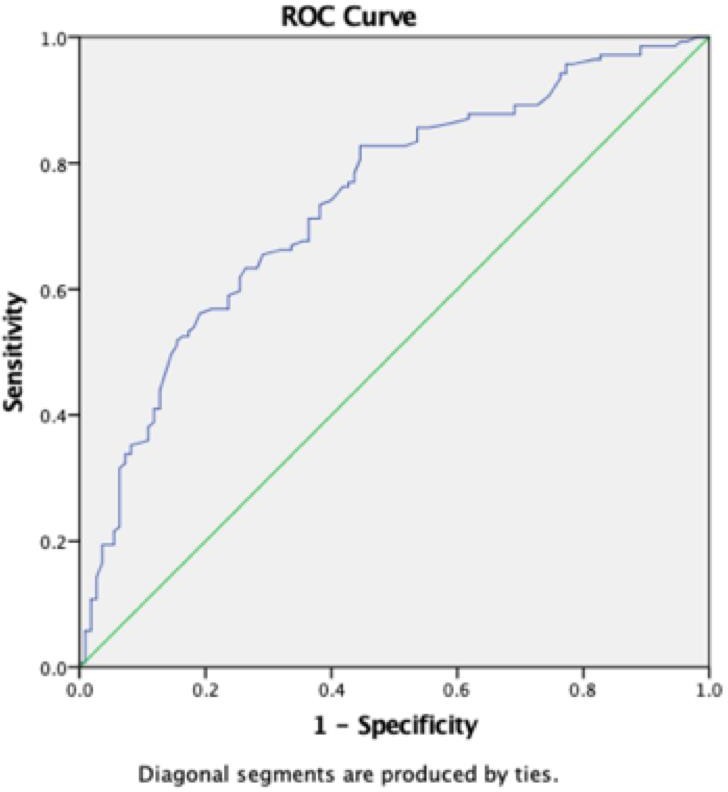
A RET-He cut-off value of 30.40 pg was derived from our study population with an area under curve of 0.739 as shown in figure 2. The curve shows sensitivity and specificity of the RET-He at different thresholds for the identification of CRA patients with ID in a diverse oncology patient population.
Role of RET-He in the Evaluation of Iron Deficiency (ID) in CRA
The statistical analysis was performed using (A) biochemical standards for ID i.e. serum iron less than 40 μg/dL, transferrin saturation less than 20% as well as (B) us in house standards i.e serum iron less than 40 μg/dL, transferrin saturation less than 30% with a RET-He cut-off at 30.4 pg. The PPV was calculated at 70.67 % and 75.9% for A & B respectively. The results were statistically significant in both (A) and (B) i.e. P value <0.001 as per table 2.
Comparative Role of RET-He <31 pg *in the evaluation of Iron Deficiency (ID) in CRA patients
Comparative role of RET-He <31 pg in the Evaluation of Iron Deficiency (ID) in CRA patients using the RET-He cut-off value established by Peerschke et al is shown in table 3.15 The statistical analysis was performed using (A) biochemical standards for ID i.e. serum iron < 40 μg/dL, TSAT < 20% as well as (B) us in house standards i.e serum iron < 40 μg/dL, transferrin saturation < 30%. With a RET-He cut-off at 31 pg, the PPV was calculated at 70 % and 74.8% for A & B respectively. The results were statistically significant in both (A) and (B) i.e. P value <0.001.
Comparative role of RET-He <32 pg in the Evaluation of Iron Deficiency (ID) in CRA patients
Comparative role of RET-He <32 pg in the Evaluation of Iron Deficiency (ID) in CRA patients using the RET-He cut-off value established by Peerschke et al is shown in table 4.15 The statistical analysis was performed using (A) biochemical standards for ID i.e. serum iron less than 40 μg/dL, transferrin saturation less than 20% as well as (B) our in house standards i.e serum iron less than 40 μg/dL, transferrin saturation less than 30%.With a RET-He cut-off at 32 pg, the PPV was calculated at 69.7 % and 73.9% for A & B respectively. The results were statistically significant in both (A) and (B) i.e. P value <0.001.
Discussion
Anemia is prevalent in 30% to 90% of patients with malignancies particularly those receiving chemotherapeutic agents [18]. The incidence and initial presentation of CRA varies depending on the type of malignancy, stage, duration of the disease, intensity and type of tumor therapy regimen, and the occurrence of intercurrent infections or surgery [16-17].
Compared to the gold standard bone marrow iron estimation, RET-He is proven to be faster, easier, more standardized and economical without any additional blood sample requirements, invasive bone marrow aspiration/biopsy procedure or technical intervention [2,6,7,15].
A standardized cut-off value of RET-He or CHr for the identification of IDA or therapeutic response has not been established yet which may be due to the variability in the studied patient population. There is also sparsity of published literature on the utility of RET-He in the evaluation of CRA globally.
In the adult population, various cut-off level for RET-He at <27.2 pg 3, <30.5 pg 4 and <30.6 pg 5 have been demonstrated, respectively with a very good sensitivity and specificity to determine iron deficient erythropoiesis. In 2014, the first ever study on the “use of RET-He to evaluate anemia in patients with cancer” was published by Peerschke et al. It reduced unnecessary iron studies by 66% in their setting and provided “rapid diagnostic information” when the automated CBC & reticulocyte counts were reported [15].
Peerschke et al calculated a RET-He threshold of 31 pg, 32 pg in their study, representing the 34th percentile of RET-He values in the study population [15]. Similarly, in our study population, with serum iron less than 40 μg/dL, TSAT less than 20% (standard definition), a RET-He cut-off value of 30.4 pg was calculated using a ROC curve (Figure 2).
Using this cut-off value of 30.4 pg, statistical analysis was performed on our study subpopulation presenting with ID (serum iron less than 40 μg/dL, TSAT less than 20%) to ascertain the role of RET-He. A PPV of 70.67 % & NPV of 61.2% was seen with 67.4% sensitivity & 64.5% specificity. When all these values were simultaneously analyzed using us in house cut-off values (i.e serum iron less than 40 μg/dL, TSAT less than 30%) the PPV was higher at 75.9% with 58.6% NPV, 67.8% sensitivity & 68% specificity (Table 2).
In the study by Peerschke et al, despite excellent negative predictive values, the corresponding specificities for each RET-He cut-off were low (i.e for RET-He < 31 pg, NPV was 94.8% with 83% specificity and for <32 pg NPV was 94.9% with 75% specificity). The RET-He cut-off values established by Peerschke et al (i.e. 31 & 32 pg) were applied to our study subpopulation (Table 3,4) and statistical analysis was performed. We found that with increasing RET-He cut-off value, our sensitivity & NPV showed an increase but the PPV simultaneously decreased. This is in concordance with their study results.
However, in the study by Peershke et al, subpopulation of IDA patients included only 23 subjects out of a total study population of 209 subjects whose population characteristics were unknown. Also, their study does not clarify whether the results were statistically significant for either of the suggested RET-He cut-off values (i.e. <31 pg or <32 pg). In contrast, our subpopulation was larger as it included 249 iron deficient subjects out of a total of 350 and our results were statistically significant (P value <0.05), thereby making this study more clinically meaningful in CRA patients.
A clinical trial titled “Study of the Interest of the Hemoglobin Content of Reticulocytes (RET-He) in the Management of Functional Military-deficient Anemia in the Patient with a Solid Tumor” at the Institut de Cancérologie de l’Ouest, France has completed recruitment the results of which are awaited. This trial is aimed at refining the current definition of functional iron deficiency in order to best adapt the iron prescription in oncology by giving iron only if necessary, i.e. if the RET-He is low [19].
In our study, we compared two sets of evaluation limits for iron deficiency (ID) and conducted a detailed cost analysis to assess their effectiveness. The first set of limits, known as the biochemical standards, includes a serum iron level of less than 40 μg/dL and a transferrin saturation (TSAT) level of less than 20%. These standards are commonly used but have cost implications. With the biochemical standards, additional iron biochemistry studies are often required to confirm the diagnosis of ID, resulting in extra laboratory tests and increased costs. Moreover, performing these studies necessitates additional blood samples to be drawn from the patients, adding to the overall cost and potentially causing delays in diagnosis and treatment initiation.
In contrast, our in-house standards propose a serum iron level of less than 40 μg/dL and a TSAT level of less than 30% as the evaluation limits for ID. This alternative approach aims to streamline the diagnostic process and improve cost effectiveness. By using our in-house standards, the need for additional iron biochemistry studies can be minimized, reducing the associated costs. Furthermore, the number of blood samples required for diagnostic purposes can be decreased, resulting in additional cost savings. This approach provides cost efficacy to patients, especially in healthcare settings with limited resources, by alleviating financial burdens and expediting the diagnostic process.
Overall, our cost analysis indicates that our in-house standards for ID evaluation offer potential cost savings and improved cost effectiveness compared to the traditional biochemical standards. By reducing the need for additional tests and sample draws, our approach can optimize resources and expedite the diagnostic process. However, it is important to note that while cost considerations are significant, clinical judgment and individualized patient assessment should always guide the decision-making process regarding the initiation of iron therapy.
RET-He results are concomitantly generated by automated hematology analyzers at no extra cost while ordering a CBC. From our study results, we were able to establish RET-He cut-off value of 30.4 pg at our institution for cancer patients with a PPV of 70%-76% in CRA patients. Due to a high incidence of anemia in cancer patients, integration of this parameter as a surrogate marker of IDA into the diagnostic algorithm can prove efficient in the rapid assessment of ID in the oncology practice without the requirement of iron biochemistry studies, additional sample draws & by providing cost efficacy to the patient. Our study is not only the first of its kind to be performed in India, but also the second in the world in large patient subset establishing the role of RET-He in the evaluation and management of CRA as per our knowledge. Major limitations of this study are that it is a non-randomized study, gold standard for assessing iron deficiency i.e. bone marrow estimation of iron stores has not been done. Further studies are warranted to enhance the understanding of the comparative cost-effectiveness between the two sets of evaluation limits for iron deficiency (ID). These studies should include a comprehensive analysis of the direct and indirect costs associated with each approach, taking into account factors such as laboratory expenses, additional sample draws, healthcare utilization, and patient outcomes. Additionally, research should focus on evaluating the potential impact of the proposed limits on the delay of iron therapy initiation in specific cases. By addressing these aspects, future studies can provide valuable insights and evidence-based recommendations for optimizing the diagnostic algorithm and resource allocation in the management of ID in cancer patients.
Summary
RET-He is a readily available parameter on automated hematology analyzers with no additional cost and effort. Hence, we recommend the incorporation of RET-He in the diagnostic algorithm for the rapid assessment of IDA in the oncology practice as a surrogate marker with cut-off value <30.4 pg which had a PPV of 75%.This will negate the requirement of iron biochemistry studies in CRA patients, expedite the process of anemia correction with I.v. iron therapy and further reduce the requirement of additional blood sampling, along with providing cost efficacy to the patient especially in the cost-limited healthcare settings. In case of non-responders, a further extensive work up should be performed to delineate the etiology of CRA.
- Brugnara C, Laufer MR, Friedman AJ, Bridges K, Platt O (1994) Reticulocyte hemoglobin content (CHr): early indicator of iron deficiency and response to therapy. Blood 83: 3100.
- Toki Y, Ikuta K, Kawahara Y, Niizeki N, Kon M, Enomoto M et al. (2017) Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of irondeficiency. Int J Hematol 106: 116-25.
- Brugnara C, Schiller B, Moran J (2006) Reticulocyte hemoglobin equivalent (RET-He) and assessment of iron-deficient states. Clinical Laboratory Hematology 28: 303-8.
- Garzia M, Di Mario A, Ferraro E, Tazza L, Rossi E, Luciani G et al. (2007) Reticulocyte hemoglobin equivalent: An indicator of reduced iron availability in chronic kidney diseases during Erythropoietin therapy. Laboratory Hematology 13: 6-11.
- Buttarello M, Pajola R, Novello E, Rebeschini M, Cantaro S, Oliosi F et al. (2010) Diagnosis of iron deficiency in patients undergoing hemodialysis. Am J Clin Pathol 133: 949-54.
- Organization WH (2011) Haemoglobin concentrations for the diagnosis of aneamia and assessment of severity: World Health Organization 1: 1-6
- Uçar MA, Falay M, Dağdas S, Ceran F, Urlu SM, Özet G (2019) The Importance of RET-He in the Diagnosis of Iron Deficiency and Iron Deficiency Anemia and the Evaluation of Response to Oral Iron Therapy. J Med Biochem 38: 496-502.
- Karlsson T (2011) Comparative evaluation of the reticulocyte hemoglobin content assay when screening for iron deficiency in elderly anemic patients. Anemia 925907.
- Joosten E, Lioen P, Brusselmans C, Indevuyst C, Boeckx N et al. (2013) Is analysis of the reticulocyte haemoglobin equivalent a useful test for the diagnosis of iron deficiency anaemia in geriatric patients? Eur J Int Med 24: 63-6.
- Torsvik IK, Markestad T, Ueland PM, Ceran F, Urlu S, Özet G et al. (2013) Evaluating iron status and the risk of anemia in young infants using erythrocyte parameters. Pediatr Res 73: 214–20.
- Schoorl M, Schoorl M, van der Gaag D, Bartels PC (2012) Effects of iron supplementation on red blood cell hemoglobin content in pregnancy. Hematol Rep 4: e24.
- Semmelrock MJ, Raggam RB, Amrein K, Avian A, Schallmoser K, Lanzer G et al. (2012) Reticulocyte hemoglobin content allows early and reliable detection of functional iron deficiency in blood donors. Clin Chim Acta 413: 678-82.
- Tiwari AK, Bhardwaj G, Arora D, Aggarwal G, Pabbi S, Dara RC (2018) Applying newer parameter RET-He to assess latent iron deficiency in blood donors-study at a tertiary care hospital in India. Vox Sang 113: 639-46.
- Miwa N, Akiba T, Kimata N Hamaguchi Y, Arakawa Y, Tamura T et al. (2010) Usefulness of measuring reticulocyte hemoglobin equivalent in the management of haemodialysis patients with iron deficiency. Int J Lab Hematol 32: 248-55.
- Peerschke EI, Pessin MS, Maslak P (2014) Using the hemoglobin content of reticulocytes (RET-He) to evaluate anemia in patients with cancer. Am J Clin Pathol 142: 506-12.
- Caro JJ, Salas M, Ward A, Goss G (2001) Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 91: 2214-21.
- Mercadante S, Gebbia V, Marrazzo A, Filosto S (2000) Anaemia in cancer. Pathophysiology and treatment. Cancer treatment reviews 26: 303-11.
- Knight K, Wade S, Balducci L (2004) Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 116: 11S-26.
- Interest of Hemoglobin Count of Reticulocytes in Management of Functional Anemia for Patient with Solid Tumor. - ClinicalTrials.gov [Internet]. Clinicaltrials.gov. 2020 [cited 16 August 2020].

Tables at a glance
Figures at a glance