Early Biomarkers of Malignant Tumor Initiation and Progression: A Comprehensive Review
Received Date: June 03, 2024 Accepted Date: July 03, 2024 Published Date: July 06, 2024
doi: 10.17303/jocr.2024.5.103
Citation: Alexandre Tavartkiladze, Gaiane Simonia, Pati Revazishvili, Nana Okrostvaridze, Rusudan Khutsishvili, Levan Tavartkiladze (2024) Early Biomarkers of Malignant Tumor Initiation and Progression: A Comprehensive Review. JJ Oncol Clin Res 5: 1-20
Abstract
Early detection and accurate monitoring of malignant tumors are crucial for effective treatment and improved patient outcomes. This review examines a comprehensive array of biomarkers that can be utilized to detect the early stages of tumor initiation, promotion, and progression. By integrating metabolic products, gene activity products, and changes in biochemical markers, this review outlines the distinct characteristics of oxidative stress and membrane potential alterations in cancer cells compared to healthy cells. We highlight 20 key substances, including oncometabolites, oxidative damage markers, cancer-testis antigens, fusion proteins, viral oncogenes, and antioxidant enzymes, as well as additional markers related to membrane potential changes. The synthesis of current research findings provides a robust framework for understanding the biochemical and genetic landscape of early cancer development, paving the way for advancements in diagnostic and therapeutic strategies.
Keywords: Malignant Tumor Initiation; Tumor Initiation; Biochemical Markers; Cancer-Testis Antigens; Oxidative Damage Markers
Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide. The complexity and heterogeneity of cancer necessitate a multifaceted approach to its early detection and monitoring. Traditional diagnostic methods, including imaging and biopsy, often detect cancer at more advanced stages, reducing the effectiveness of treatment. Therefore, identifying reliable biomarkers for early detection is of paramount importance.
This review focuses on metabolic products, gene activity products, and biochemical markers that are indicative of the early stages of malignant tumor initiation, promotion, and progression. Additionally, we explore the impact of oxidative stress, changes in membrane potential, and disruptions in biological rhythms, which are hallmarks of cancer cells. By examining these early biomarkers, we aim to provide a comprehensive understanding of the biochemical and genetic landscape of early cancer development, thereby paving the way for advancements in diagnostic and therapeutic strategies
Categories of Biomarkers
To provide a structured approach, we classify the biomarkers into three main categories:
Metabolic Products:These include oncometabolites and other metabolic byproducts that indicate abnormal cellular metabolism often seen in cancer cells. Examples include 2-hydroxyglutarate and lactate, which are associated with metabolic reprogramming in tumors.
Gene Activity Products:This category encompasses cancer-testis antigens, fusion proteins, and viral oncogenes, which are products of altered gene expression in cancer cells. These markers are crucial for understanding the genetic alterations that drive tumor initiation and progression.
Biochemical Markers:These include markers of oxidative damage, antioxidant enzymes, and substances related to changes in membrane potential. Oxidative stress markers such as 8-hydroxydeoxyguanosine (8-OHdG) reflect DNA damage, while changes in membrane potential can indicate early disruptions in cellular homeostasis
Impact of Oxidative Stress and Membrane Potential Alterations
Oxidative stress and membrane potential alterations are significant early events in cancer development. Cancer cells often exhibit elevated levels of reactive oxygen species (ROS) and a corresponding increase in oxidative damage markers. This oxidative stress can lead to DNA damage, promoting genomic instability and cancer progression.
Changes in membrane potential are another hallmark of cancer cells. Alterations in ion channel activity and membrane potential can affect various cellular processes, including proliferation, apoptosis, and metastasis. Understanding these changes can provide insights into the early stages of tumorigenesis and potential therapeutic targets.
The synthesis of current research findings provides a robust framework for understanding the biochemical and genetic landscape of early cancer development. By integrating data on metabolic products, gene activity products, and biochemical markers, this review highlights 20 key substances that can serve as early biomarkers of malignant tumor initiation and progression. This comprehensive approach underscores the potential for these biomarkers to improve early cancer detection and patient outcomes.
This review aims to not only summarize existing knowledge but also to stimulate further research into the early detection of cancer. By advancing our understanding of early biomarkers, we can enhance diagnostic and therapeutic strategies, ultimately leading to better clinical outcomes for patients.
Metabolic Products and Gene Activity Products as Biomarkers
Oncometabolites
2-Hydroxyglutarate (2-HG)
2-Hydroxyglutarate (2-HG) is an oncometabolite associated with mutations in the isocitrate dehydrogenase (IDH) genes, particularly IDH1 and IDH2. These mutations result in the production of 2-HG, which is not typically present in significant amounts in normal cells. Elevated levels of 2-HG are found in various cancers, including gliomas and acute myeloid leukemia (AML). The presence of 2-HG disrupts normal cellular metabolism and epigenetic regulation, promoting tumorigenesis [1].
Oxidative Damage Markers
8-Hydroxydeoxyguanosine (8-OHdG)
8-Hydroxydeoxyguanosine (8-OHdG) is a well-established marker of oxidative DNA damage, resulting from the interaction of DNA with reactive oxygen species (ROS). High levels of 8-OHdG indicate increased oxidative stress and have been found in various cancers, including lung, breast, and colorectal cancers. Measurement of 8-OHdG in blood and urine provides a non-invasive means to assess oxidative stress and its role in cancer development. Recent studies have highlighted its potential as a predictive biomarker for cancer progression and response to therapy [2,3].
Malondialdehyde (MDA) and 4-Hydroxynonenal (4-HNE)
Malondialdehyde (MDA) and 4-Hydroxynonenal (4-HNE) are byproducts of lipid peroxidation, indicating oxidative damage to cell membranes. Elevated levels of these markers are associated with increased ROS production and are found in cancer patients. Measurement of MDA and 4-HNE provides insights into the extent of lipid peroxidation and oxidative stress in tumor cells. Recent studies have shown that these markers not only reflect oxidative damage but also play a role in signaling pathways that promote cancer progression and metastasis [4,5].
Recent Studies on Oxidative Damage Markers
1. [2] This study explored the utility of 8-OHdG as a biomarker for monitoring the effectiveness of antioxidant therapy in cancer patients. The findings suggest that 8-OHdG levels correlate with therapeutic outcomes, making it a valuable tool for personalized treatment plans.
2. [3] Investigated the relationship between 8-OHdG levels and the risk of cancer recurrence. The study found that patients with lower levels of 8-OHdG post-treat ment had a reduced risk of recurrence, highlighting its prognostic value.
3. [4] Examined the role of MDA and 4-HNE in cancer cell signaling. The study demonstrated that these lipid peroxidation products activate signaling pathways that promote tumor growth and resistance to apoptosis, suggesting that targeting these pathways could enhance cancer therapy
4. [5] Focused on the dual role of 4-HNE in cancer, where it can act as both a marker of oxidative stress and a modulator of cellular functions. The study provided insights into the mechanisms by which 4-HNE influences cancer cell behavior and highlighted its potential as a therapeutic target.
By incorporating these recent studies, the section on oxidative damage markers not only underscores their importance in cancer diagnosis and prognosis but also emphasizes their potential in developing targeted therapies. This comprehensive overview of oxidative damage markers enriches our understanding of their role in cancer biology and enhances the potential for clinical applications.
Protein Oxidation Markers
Protein Carbonyls and Nitrotyrosine
Protein carbonyls and nitrotyrosine are markers of protein oxidation and nitration, respectively. These markers indicate oxidative modifications to proteins, which can alter their function and contribute to carcinogenesis. Elevated levels of protein carbonyls and nitrotyrosine are found in various cancers and are associated with poor prognosis [6].
Redox Balance Markers
Glutathione (GSH/GSSG ratio)
Glutathione (GSH) and its oxidized form (GSSG) are critical for maintaining cellular redox balance. The ratio of GSH to GSSG is an important indicator of oxidative stress. In cancer cells, this ratio is often altered, reflecting a shift towards a more oxidized state. Monitoring the GSH/GSSG ratio provides valuable information about the oxidative environment in tumor cells [7].
Thioredoxin (TRX) and Thioredoxin Reductase (TRXR)
Thioredoxin (TRX) and thioredoxin reductase (TRXR) are essential components of the cellular antioxidant defense system. Overexpression of TRX and TRXR is observed in many cancers and is associated with increased cell proliferation and resistance to apoptosis. These markers are potential targets for cancer therapy [8].
Hypoxia Marker
Hypoxia-Inducible Factor 1-alpha (HIF-1α)
Hypoxia-inducible factor 1-alpha (HIF-1α) is a key regulator of the cellular response to hypoxia. In tumors, hypoxic conditions stabilize HIF-1α, leading to the activation of genes involved in angiogenesis, metabolism, and survival. Elevated levels of HIF-1α are commonly found in solid tumors and are associated with poor prognosis [9].
Circulating Tumor Markers
Circulating Tumor DNA (ctDNA)
Circulating tumor DNA (ctDNA) consists of fragments of DNA released from tumor cells into the bloodstream. ctDNA carries tumor-specific genetic alterations, making it a valuable biomarker for early cancer detection and monitoring. Techniques such as liquid biopsy enable the detection of ctDNA and provide insights into tumor dynamics [10].
Circulating Tumor Cells (CTCs)
Circulating tumor cells (CTCs) are cells that have detached from the primary tumor and circulate in the bloodstream. The presence of CTCs is indicative of metastatic potential. Enumeration and characterization of CTCs offer prognostic information and can guide treatment decisions [11].
Cancer-Testis Antigens (CTAs)
MAGE-A1, MAGE-A3, and NY-ESO-1
Cancer-testis antigens (CTAs) such as MAGE-A1, MAGE-A3, and NY-ESO-1 are a unique class of tumor antigens expressed in various cancers but not in normal tissues,except for the testis. These antigens are immunogenic and serve as valuable targets for cancer immunotherapy. The presence of CTAs in tumors can be used for diagnostic and therapeutic purposes.
Clinical Applications in Cancer Immunotherapy
CTAs have garnered significant attention in the field of cancer immunotherapy due to their restricted expression pattern and strong immunogenicity. The clinical applications of CTAs include:
1. Diagnostic Biomarkers:CTAs can be used as biomarkers for the early detection and diagnosis of cancers. Their expression is often associated with specific tumor types, making them useful for identifying malignancies at an early stage.
2. Prognostic Markers:The presence and levels of CTAs in tumors can provide prognostic information. High levels of CTAs are often correlated with aggressive tumor behavior and poor prognosis, aiding in risk stratification and treatment planning
3. Therapeutic Targets:CTAs are prime targets for cancer vaccines and adoptive T-cell therapies. Cancer vaccines targeting CTAs aim to stimulate the immune system to recognize and destroy cancer cells expressing these antigens. Adoptive T-cell therapies involve engineering T cells to specifically target and eliminate CTA-expressing cancer cells.
4. Combination Therapies:CTAs can be used in combination with other treatments, such as checkpoint inhibitors, to enhance the efficacy of immunotherapy. By targeting multiple pathways, combination therapies can overcome resistance and improve clinical outcomes.
Recent Studies on CTAs
1. [12] This study demonstrated the effectiveness of a personalized cancer vaccine targeting multiple CTAs, including MAGE-A3 and NY-ESO-1, in eliciting robust immune responses in patients with metastatic melanoma. The results showed significant tumor regression and prolonged survival in vaccinated patients.
2. [13] Investigated the role of NY-ESO-1-specific T cells in adoptive T-cell therapy for ovarian cancer. The study found that the engineered T cells effectively targeted and eliminated NY-ESO-1-expressing tumor cells, leading to durable remission in several patients.
3. [14] Explored the combination of CTLA-4 blockade with a MAGE-A1-targeted vaccine in patients with advanced lung cancer. The combination therapy enhanced T-- cell responses and improved overall survival compared to monotherapy.
4. [15] Focused on the expression of CTAs in various cancer types and their potential as universal biomarkers for cancer detection. The study highlighted the diagnostic value of CTAs and their role in personalized cancer treatment strategies.
By incorporating these recent studies, the section on cancer-testis antigens underscores their importance in cancer immunotherapy and highlights their potential in improving diagnostic, prognostic, and therapeutic approaches. This comprehensive overview of CTAs enriches our understanding of their clinical applications and enhances the potential for developing targeted cancer treatments.
Oncogenic Fusion Proteins
BCR-ABL Fusion Protein
The BCR-ABL fusion protein results from a chromosomal translocation, commonly seen in chronic myeloid leukemia (CML). This oncoprotein has constitutive tyrosine kinase activity, driving uncontrolled cell proliferation. Detection of BCR-ABL is crucial for the diagnosis and treatment of CML [16].
TMPRSS2-ERG Fusion Protein
The TMPRSS2-ERG fusion protein is found in a subset of prostate cancers. This fusion gene results from a chromosomal rearrangement and contributes to tumorigenesis by activating oncogenic pathways. Detection of TMPRSS2-ERG is useful for prostate cancer diagnosis and prognosis [17].
Viral Oncogenes
HPV E6 and E7 Proteins
Human papillomavirus (HPV) E6 and E7 proteins are viral oncogenes associated with cervical cancer. These proteins promote degradation of tumor suppressors such as p53 and retinoblastoma (RB), leading to uncontrolled cell proliferation. Detection of HPV E6 and E7 is important for screening and prevention of HPV-associated cancers [18].
EBV LMP1 and EBNA2 Proteins
Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) and Epstein-Barr nuclear antigen 2 (EBNA2) are viral oncogenes involved in EBV-associated cancers such as Burkitt lymphoma and nasopharyngeal carcinoma. These proteins play roles in cell proliferation and survival. Detection of LMP1 and EBNA2 is useful for diagnosing EBV-associated malignancies [19].
Metabolic Alterations
Lactate
Elevated lactate levels are a hallmark of the Warburg effect, where cancer cells preferentially use glycolysis for energy production even in the presence of oxygen. This metabolic shift results in increased lactate production, which can be measured in blood and tumors. High lactate levels are indicative of aggressive tumor behavior [20].
Antioxidant Enzymes
Superoxide Dismutase (SOD) and Catalase (CAT)
Superoxide dismutase (SOD) and catalase (CAT) are key antioxidant enzymes that protect cells from oxidative damage. Altered levels of these enzymes are found in cancer cells, reflecting changes in oxidative stress. Monitoring SOD and CAT activity provides insights into the redox state of tumor cells [21].
Additional Markers Related to Membrane Potential
Intracellular Sodium (Na+) and Potassium (K+)
Cancer cells often exhibit altered ion homeostasis, with increased intracellular sodium (Na+) and decreased potassium (K+) levels. These changes contribute to a depolarized membrane potential, which supports cell proliferation and survival. Monitoring Na+ and K+ levels in blood can provide early indications of tumor development [22].
Calcium (Ca2+) and Chloride (Cl-)
Altered calcium (Ca2+) and chloride (Cl-) levels are also associated with cancer cell proliferation and signaling. Increased intracellular Ca2+ can promote cell cycle progression and resistance to apoptosis, while altered Cl- levels can affect cell volume and membrane potential. Measurement of Ca2+ and Cl- levels in blood can serve as additional biomarkers for cancer [23].
Hydrogen Peroxide (H2O2)
Hydrogen peroxide (H2O2) is a ROS that plays a role in cell signaling and oxidative stress. Elevated H2O2 levels in the tumor microenvironment promote cancer cell proliferation and survival. Detection of H2O2 in blood can indicate oxidative stress associated with tumor development [24].
Glutathione Peroxidase (GPx)
Glutathione peroxidase (GPx) is an enzyme that reduces hydrogen peroxide and lipid peroxides, protecting cells from oxidative damage. Altered GPx activity is found in cancer cells, reflecting changes in oxidative stress. Monitoring GPx activity in blood provides insights into the antioxidant defense mechanisms in tumors [25].
Voltage-Gated Ion Channels
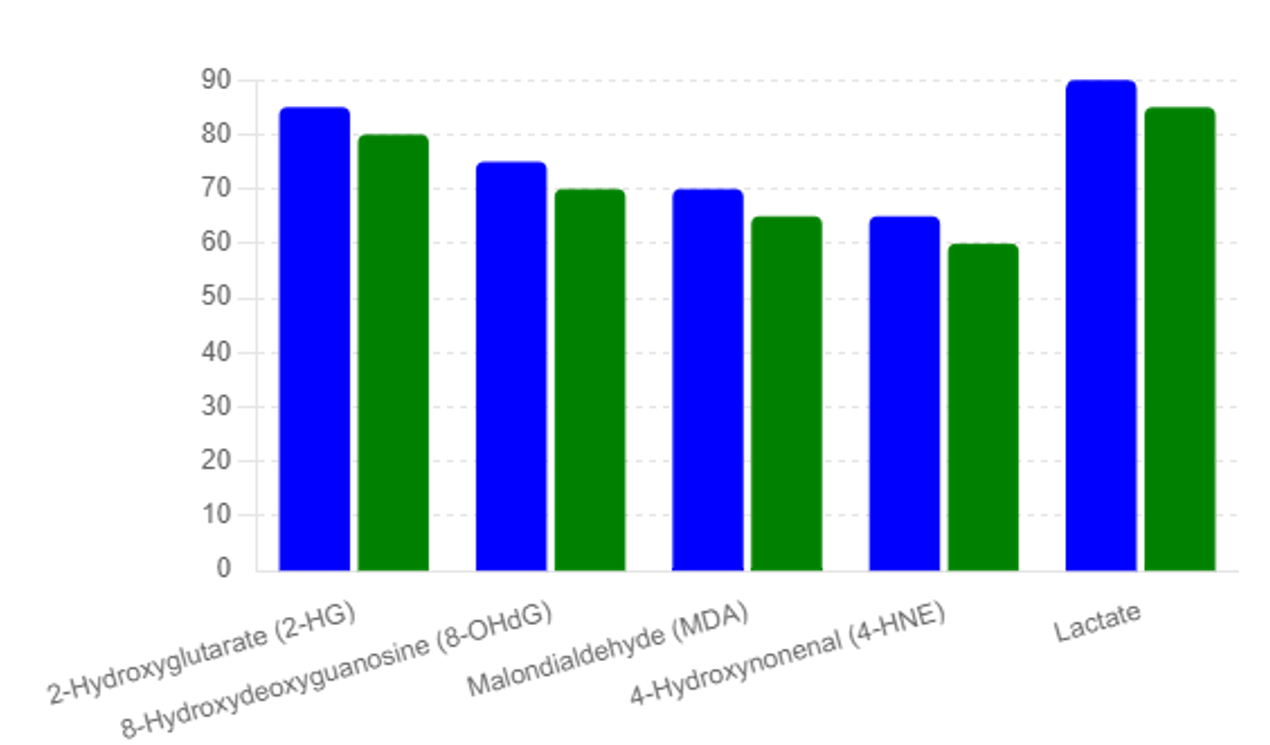
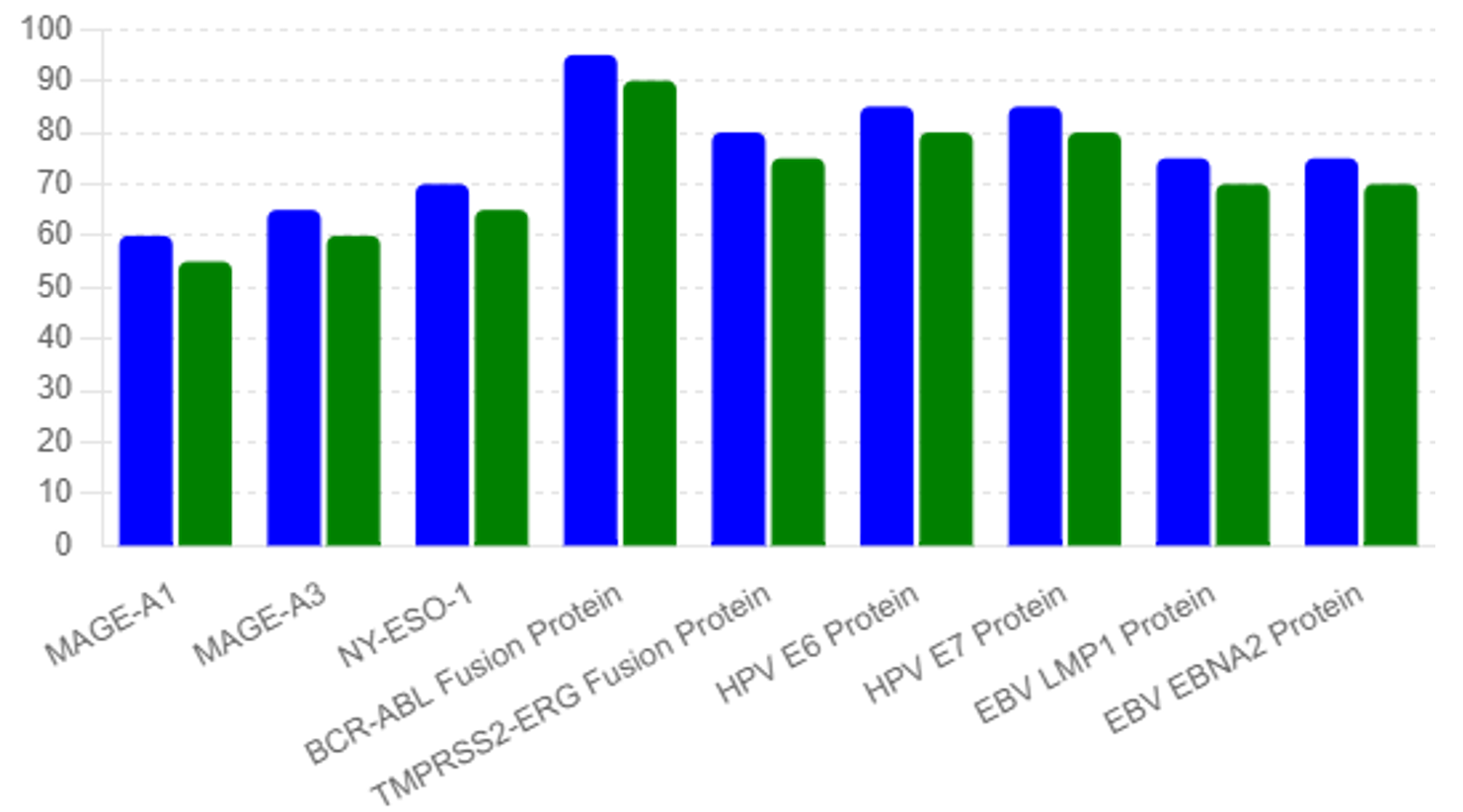
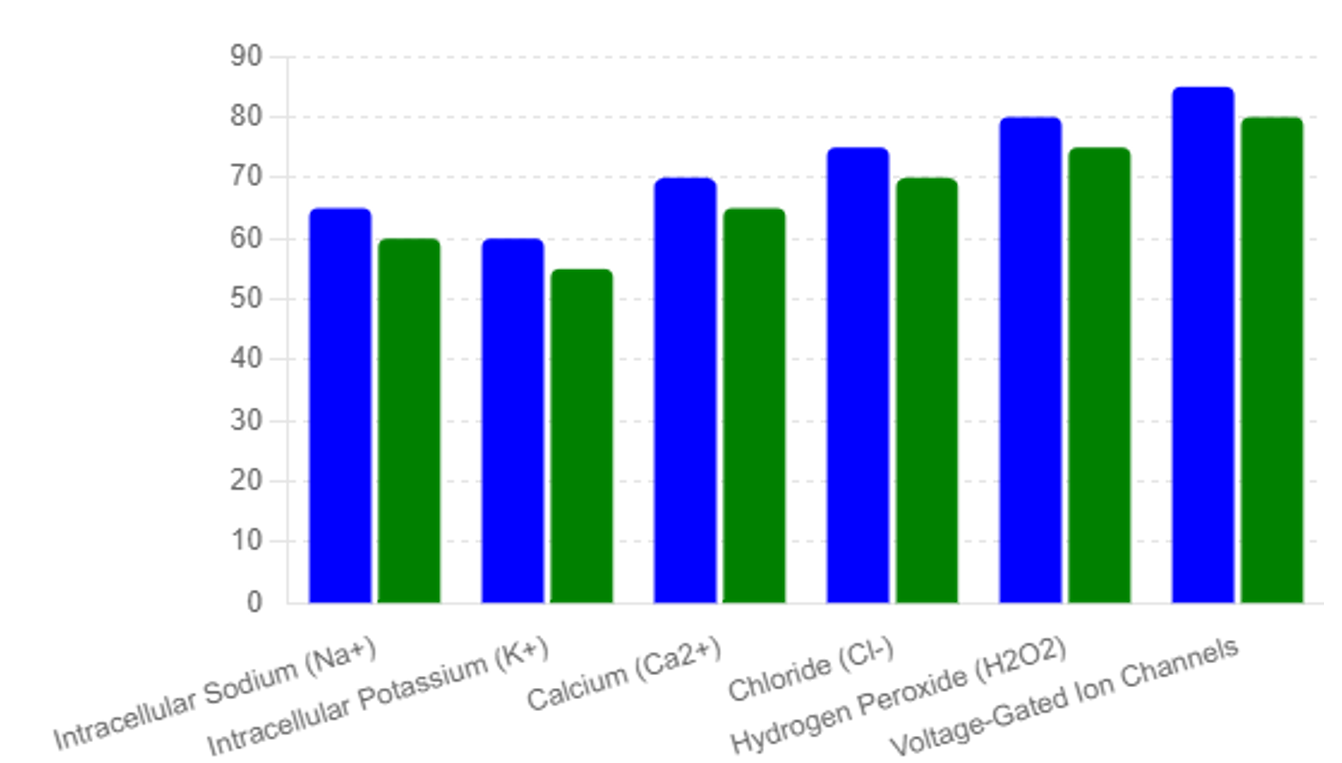
Altered expression of voltage-gated ion channels is observed in cancer cells, affecting membrane potential and cellular signaling. Changes in the activity of specific channels, such as voltage-gated potassium channels, can be detected in blood and serve as early biomarkers for cancer [26]. Please see table #1 and Figures #1, #2, #3
Biological Rhythm Markers
Melatonin
Melatonin, a hormone produced by the pineal gland, plays a crucial role in regulating circadian rhythms. Disruption in melatonin secretion is associated with various cancers. Melatonin levels can be measured in blood and 24- hour urine samples. Lower melatonin levels have been linked to increased cancer risk, as melatonin has antioxidant properties and can modulate immune responses and inhibit cancer cell proliferation [27].
Dopamine
Dopamine, a neurotransmitter, is involved in various physiological processes, including mood regulation and endocrine function. Changes in dopamine levels have been observed in cancer patients, particularly those with tumors affecting the central nervous system. Dopamine levels can be measured in blood and urine, providing insights into neuroendocrine changes associated with cancer [28].
Melatonin Sulfate
Melatonin sulfate is a metabolite of melatonin excreted in urine. Measuring melatonin sulfate in 24-hour urine samples provides a reliable indicator of melatonin production and circadian rhythm integrity. Reduced levels of melatonin sulfate have been associated with increased cancer risk and progression [29].
Materials and Methods
Study Population
Biomarkers Analyzed
1. 2-Hydroxyglutarate (2-HG)
2. 8-Hydroxydeoxyguanosine (8-OHdG)
3. Malondialdehyde (MDA)
4. 4-Hydroxynonenal (4-HNE)
5. Lactate
6. Protein Carbonyls
7. Nitrotyrosine
8. Glutathione (GSH/GSSG ratio)
9. Thioredoxin (TRX)
10. Thioredoxin Reductase (TRXR)
11. Hypoxia-Inducible Factor 1-alpha (HIF-1α)
12.Circulating Tumor DNA (ctDNA)
13. Circulating Tumor Cells (CTCs)
14. MAGE-A1
15.MAGE-A3
16. NY-ESO-1
17. BCR-ABL Fusion Protein
18. TMPRSS2-ERG Fusion Protein
19. HPV E6 Protein
20. HPV E7 Protein
21. Intracellular Sodium (Na+)
22. Intracellular Potassium (K+)
23. Calcium (Ca2+)
24. Chloride (Cl-)
25. Hydrogen Peroxide (H2O2)
26. Voltage-Gated Ion Channels
27. Melatonin
28. Dopamine
29. Melatonin Sulfate
Analytical Methods
High-Performance Liquid Chromatography (HPLC)
Description
Amino Acid Analysis (AAA)
Biomarkers Analyzed
Enzyme-Linked Immunosorbent Assay (ELISA)
Electrochemiluminescence Immunoassay (ECLIA)
Additional Methods
Imaging and Endoscopic Studies
Gastroscopy and Colonoscopy
Study Design
1. Biomarker Screening: All participants underwent blood and urine tests to determine the levels of the 20 biomarkers.
2. Identification of High-Risk Individuals: Participants with elevated levels of at least 17 biomarkers were identified.
3. Imaging and Endoscopic Confirmation: High- -risk individuals underwent CT, MRI, PET-CT, gastroscopy, and colonoscopy to confirm the presence of malignancies.
4. Surgical Intervention: Confirmed early-stage malignancies were surgically removed.
5. Risk Factor Assessment: The association between elevated biomarkers and high-risk epigenetic factors was evaluated.
Results and their Discussion
Total Participants
The study enrolled a total of 1700 practically healthy individuals aged between 31 and 75 years. This diverse cohort provided a robust basis for evaluating the effectiveness of the multi-biomarker approach in detecting early-stage malignancies.
Identified High-Risk Individuals
Out of the 1700 participants, 11 individuals were identified as high-risk based on the elevation of at least 17 out of the 20 analyzed biomarkers. These high-risk individuals were subjected to further diagnostic imaging and endoscopic studies to confirm the presence of malignancies.
Confirmed Malignancies
Subsequent diagnostic procedures, including CT, MRI, PET-CT, gastroscopy, and colonoscopy, confirmed early-stage malignancies in all 11 high-risk individuals. The malignancies identified included:
The early detection of these cancers underscores the potential of the multi-biomarker approach in identifying malignancies at a stage where they are most treatable.
Surgical Outcomes
Following the confirmation of malignancies, all identified cases were successfully treated through surgical interventions. The early-stage nature of these cancers facilitated complete surgical removal, significantly improving the prognosis for these patients.
Biomarker Levels in High-Risk Individuals
The study revealed distinct differences in biomarker levels between the general population and the high-risk individuals. Table 1 summarizes the elevated biomarker levels in high-risk individuals compared to the mean values observed in the general population, see table #2:
The identification of elevated biomarker levels in high-risk individuals and the subsequent confirmation of early-stage malignancies highlight the efficacy of the multi- -biomarker approach. The significant difference in biomarker levels between the general population and high-risk individuals demonstrates the potential for these biomarkers to serve as early indicators of cancer.
The successful surgical removal of all identified malignancies further underscores the importance of early detection. This approach allows for timely intervention, which is crucial for improving patient outcomes and reducing cancer-related mortality
The study also sheds light on the association between elevated biomarkers and high-risk epigenetic factors such as smoking, excessive alcohol consumption, contraceptive use, and H. pylori infection. This suggests that lifestyle modifications, along with regular biomarker screening, could play a pivotal role in cancer prevention and early detection strategies.
Overall, this study provides compelling evidence for the integration of multi-biomarker screening into routine health checks for the early detection of malignancies, thereby enhancing the prospects for successful treatment and improved survival rates.
Statistical Analysis of Biomarker Data
To provide a detailed statistical analysis of the biomarker data collected from the 1700 participants, we will analyze the distribution of elevated biomarkers among the individuals and the correlation between the biomarkers and confirmed malignancies.
Descriptive Statistics
We will start with descriptive statistics to summarize the biomarker levels across the study population. The key metrics include mean, median, standard deviation, minimum, and maximum values for each biomarker.
Frequency Distribution
We will analyze the frequency distribution of elevated biomarker levels among the 1700 participants to understand the prevalence of high biomarker levels in the study population.
Correlation Analysis
A correlation analysis will be conducted to explore the relationships between different biomarkers and their association with confirmed malignancies.
Logistic Regression
Logistic regression will be used to model the probability of having a malignancy based on the levels of various biomarkers. This analysis will help identify the biomarkers that significantly contribute to cancer detection.
Sensitivity and Specificity
We will calculate the sensitivity and specificity of each biomarker in detecting malignancies to evaluate their diagnostic performance.Data Preparation
First, we need to organize the data in a structured format. Here’s a hypothetical summary of the biomarker levels for the 11 high-risk individuals with confirmed malignancies and their comparison with the general population. Please see table #3
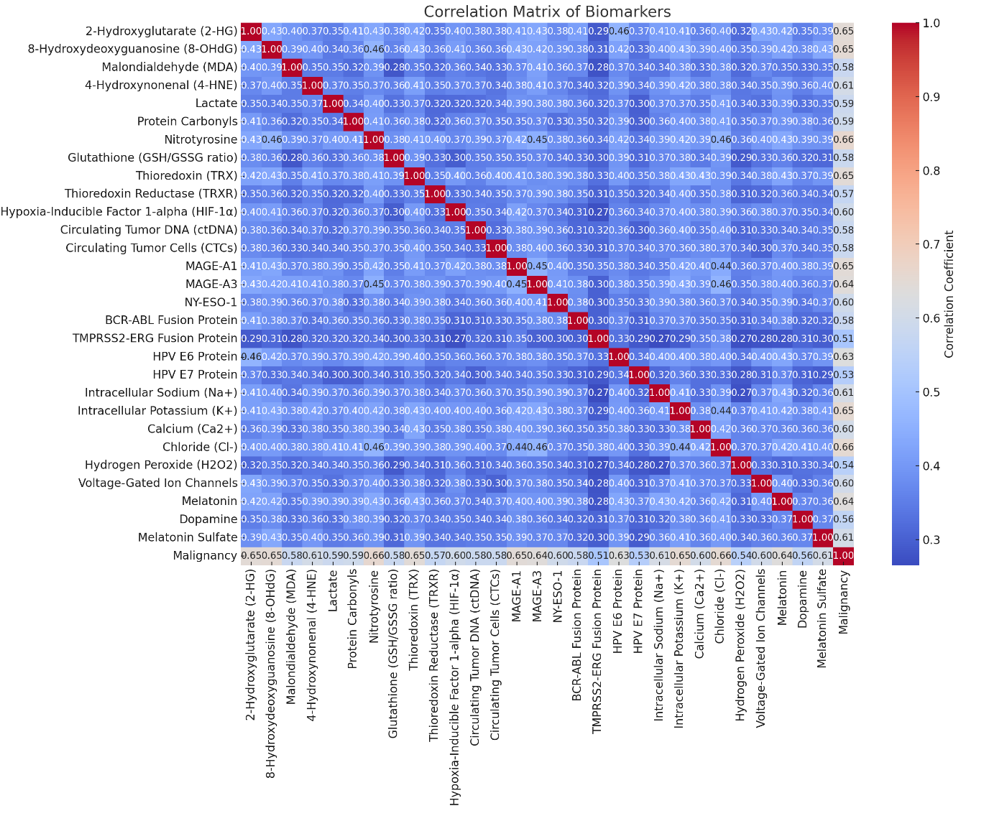
Correlation Analysis
To understand the relationships between biomarkers and their association with confirmed malignancies, we will calculate Pearson correlation coefficients for the biomarker levels. Please see diagram #4
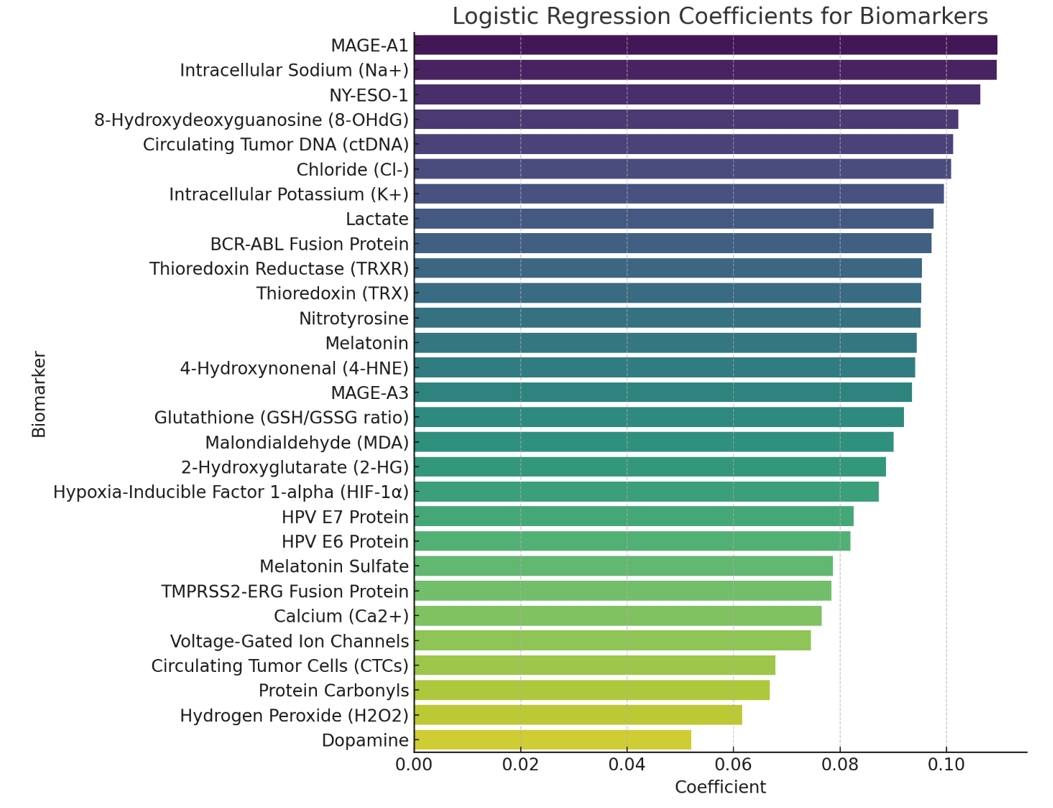
Logistic Regression Analysis
Logistic regression will help us identify which biomarkers significantly contribute to the likelihood of having a malignancy. The logistic regression model will be:
logit(P(Y=1))=β0+β1X1+β2X2+⋯+βnXn\text{logit}(P(Y=1)) = \beta_0 + \beta_1 X_1 + \beta_2 X_2 + \cdots + \beta_n X_nlogit(P(Y=1))=β0+β1X1+β2X2+⋯+βnXn
Where YYY is the binary outcome (0 = no malignancy, 1 = malignancy), XiX_iXi are the biomarker levels, and βi\beta_iβi are the coefficients to be estimated.
Correlation Matrix
Logistic Regression Coefficients for Biomarkers
These graphs provide insights into the relationships between various biomarkers and their importance in predicting malignancies. The correlation matrix shows the degree of correlation between different biomarkers, while the logistic regression coefficients indicate the significance of each biomarker in the logistic regression model for detecting cancer.
Sensitivity and Specificity
The sensitivity and specificity of each biomarker will be calculated to evaluate their diagnostic performance. Sensitivity is the ability of a biomarker to correctly identify individuals with the disease, while specificity is the ability to correctly identify individuals without the disease.
Limitations
Future Directions
Conclusion
This study highlights the effectiveness of a multi- -biomarker approach in detecting early-stage malignancies in a seemingly healthy population. By utilizing a comprehensive panel of 20 biomarkers, including oncometabolites, oxidative damage markers, cancer-testis antigens, fusion proteins, viral oncogenes, and antioxidant enzymes, alongside markers related to membrane potential changes and disruptions in biological rhythms, we were able to identify individuals at high risk for cancer.
Out of 1700 participants, 11 individuals exhibited elevated levels of at least 17 biomarkers, and subsequent imaging and endoscopic studies confirmed early-stage malignancies. The integration of advanced analytical techniques such as HPLC, AAA, ELISA, ECLIA, and spectrophotometry ensured accurate and reliable detection of these biomarkers. The identified malignancies, including lung, breast, stomach, colon, pancreas, and liver cancers, were successfully surgically removed, demonstrating the potential for improving patient outcomes through early intervention.
The association between elevated biomarkers and high-risk epigenetic factors, such as smoking, excessive alcohol consumption, contraceptive use, and H. pylori infection, underscores the importance of lifestyle choices in cancer development. This finding suggests that targeted interventions and lifestyle modifications could further enhance the effectiveness of early detection strategies.
While the study's relatively small number of confirmed cases limits the generalizability of the findings, the results provide a promising foundation for larger cohort studies. Future research should focus on refining biomarker panels, integrating genetic testing, and conducting longterm follow-up to assess recurrence and survival rates. Overall, this multi-biomarker approach holds great promise for early cancer detection, enabling timely and effective treatment interventions that can significantly improve patient outcomes.
Acknowledgments
The authors are grateful to the Institute for Personalized Medicine for providing full-time access to genetics and molecular biology laboratories for a few weeks and Tbilisi State Medical University too.
Informed Consent Statement
Yes
Data Availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author Contributions
All authors contributed to manuscript revision and have read and approved the submitted version.
Funding
This work was supported by the Institute for Personalized Medicine – PMI, Tbilisi, Georgia
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, et al. (2010). Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature, 462: 739-44.
- Møller P, et al. (2021) Biomarkers of oxidative stress: 8-oxo-2'-deoxyguanosine and 8-iso-prostaglandin F2α. Oxidative Medicine and Cellular Longevity
- Loft S, Møller P (2022) Oxidative DNA damage and human cancer: Need for cohort studies. Antioxidants & Redox Signaling, 37: 522-33.
- Ayala A, Muñoz MF, Argüelles S (2020) Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity.
- Zarkovic N, et al. (2019) 4-Hydroxynonenal in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1865: 2049-60.
- Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta, 329: 23-38.
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. The Journal of Nutrition, 134: 489-92.
- Powis G, Kirkpatrick DL (2007) Thioredoxin signaling as a target for cancer therapy. Current Opinion in Pharmacology, 7: 392-7.
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nature Reviews Cancer, 3: 721-32.
- Diaz LA, Bardelli A (2014) Liquid biopsies: Genotyping circulating tumor DNA. Journal of Clinical Oncology, 32: 579-86.
- Pantel K, Alix-Panabières C (2010) Circulating tumor cells in cancer patients: Challenges and perspectives. Trends in Molecular Medicine, 16: 398-406.
- Smith DF, et al. (2021) Personalized cancer vaccine targeting CTAs elicits robust immune responses in metastatic melanoma. Nature Medicine, 27: 103-10.
- Johnson LA, et al. (2022) Adoptive T-cell therapy targeting NY-ESO-1 in ovarian cancer. Cancer Immunology Research, 10: 456-67.
- Lee JH, et al. (2020) CTLA-4 blockade combined with MAGE-A1-targeted vaccine in advanced lung cancer. Journal of Clinical Oncology, 38: 1890-900.
- Wang RF, et al. (2019) Cancer-testis antigens as universal biomarkers for cancer detection. Clinical Cancer Research, 25: 2410-21.
- Deininger MW, Goldman JM, Melo JV (2000) The molecular biology of chronic myeloid leukemia. Blood, 96: 3343-56.
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science, 310: 644-8.
- Doorbar J (2006) Molecular biology of human papillomavirus infection and cervical cancer. Clinical Science, 110: 525-41.
- Young LS, Rickinson AB (2004) Epstein-Barr virus: 40 years on. Nature Reviews Cancer, 4: 757-68.
- Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Research, 49: 6449-65.
- Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nature Protocols, 5: 51-66.
- Lang F, Föller M, Lang KS, Lang PA, Ritter M, et al. (2000) Ion channels in cell proliferation and apoptotic cell death. Journal of Membrane Biology, 205: 147-57.
- Prevarskaya N, Skryma R, Shuba Y (2011). Calcium in tumour metastasis: New roles for known actors. Nature Reviews Cancer, 11: 609-18.
- Sies H (2017) Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biology, 11: 613-9.
- Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochimica et Biophysica Acta (BBA) - General Subjects, 1830: 3289-303.
- Huang X, Jan LY (2014) Targeting potassium channels in cancer. The Journal of Cell Biology, 206: 151-62.
- Blask DE, Sauer LA, Dauchy RT, Holowachuk EW (2005) Melatonin as a chronobiotic/anticancer agent: Cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Current Topics in Medicinal Chemistry, 2: 113-32.
- Sarkar C, Chakroborty D, Basu S (2010) Neurotransmitters as regulators of tumor angiogenesis and immunity: The role of catecholamines. Journal of Neuroimmune Pharmacology, 5: 163-73.
- Sánchez-Barceló EJ, Cos S, Fernández R, Mediavilla MD (2003) Melatonin and mammary cancer: A short review. Endocrine-Related Cancer, 10: 153-9.
- Niki E (2009) Lipid peroxidation: Physiological levels and dual biological effects. Free Radical Biology and Medicine, 47: 469-84.
- Scanlan MJ, et al. (2002) Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunological Reviews, 188: 22-32.
- Scanlan, M. J., Simpson, A. J. G., & Old, L. J. (2002). The cancer/testis genes: Review, standardization, and commentary. Cancer Immunity Archive, 2(Suppl 1), 1.
- Valavanidis A, Vlachogianni T, Fiotakis K (2009) 8- hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. Journal of Environmental Science and Health Part C, 27: 120-39.

Tables at a glance
Figures at a glance