Effect of Phytic Acid on the Enzymatic Activities of Starch Degrading Human Salivary α-Amylase
Received Date: March 03, 2023 Accepted Date: March 30, 2023 Published Date: April 03, 2023
doi: 10.17303/jocs.2023.1.102
Citation: Uzma Rashid (2023) Effect of Phytic Acid on the Enzymatic Activities of Starch Degrading Human Salivary α-Amylase. J Org Chem Chem Sci 1: 1-10.
Abstract
α- amylase catalyzes the hydrolysis of internal glycosidic bonds in starch and related polysaccharides into monosaccharides, and very important for human digestion. In this study, the inhibitory activity of phytic acid on salivary α-amylase was studied in various physiological conditions. Phytic acid is complex compound with six phosphate groups and found to be a potential inhibitor of human salivaryα-amylase. It completely inhibited the activity of particular enzyme at the concentration of 40 mM in neutral pH and 0.4% of substrate concentration. The type of inhibition through which phytic acid kinetically inhibit the human salivary α-amylase activity was mixed non-competitive and pH dependent. Phytic acid showed maximum inhibition of enzyme activity after 40 minutes of pre-incubation time.
Keywords:α-Salivary Amylase; Phytic Acid; Inhibition; Mixed Non-Competitive
Introduction
Starch is a polymer of repeating units of glucose with two major components amylose and amylopectin. Amylose builds upto 15–35% of the granules in most plants is a primarily linear polysaccharide with α-(1–4)-linkedD-glucose units. Amylopectin is a highly branched molecule with α-(1–4)-linked D-glucose backbones and exhibits about 5% of α-(1–6)-linked branches, which have a profound effect on the physical and biological properties of starch [1,2]. It is the main source of carbohydrate in the human diet. The α-amylase (α-1, 4-glucon-4-gluconohydrolase, EC 3.2.1.1) catalyzes the hydrolysis of internal glycosidic bonds in starch and related poly as well as oligosaccharides and widespread in all three domains of life (Archaea, Bacteria and Eucarya). Human α-amylase is present in both salivary and pancreatic secretions and plays a key role in the hydrolysis of starch in human food. Human salivary α-amylase is present in the oral cavity, provides the initial partial cleavage of starch into shorter oligomers, which are then further hydrolyzed by the pancreatic enzymes [3,4]. Metal ions usually act as cofactors for enzyme and are very important for enzymatic activities of enzymes. The chelating molecules usually inhibit the activities of many enzymes by binding with cofactor and block their availability for enzyme activity. This means of inhibiting enzyme activity serves as a major control mechanism in biological systems [5,6].
Phytic acid (Myo-Inositol Hexaphosphate) is a complex carbohydrate which has chelating property to bind the minerals [7-12]. Myo-inositol hexaphosphate is the major storage form of phosphorus in cereals, legumes and oil seeds with six phosphate groups extending from the central inositol ring and serves as an excellent chelator of minerals [13,14]. It has been reported that myo-inositol hexaphosphate strongly inhibit α-amylases of different origins [15,16]. The effect of bran phytic acid on the activity of wheat amylase was investigated and it was report that the it signficantlty effect the activity of wheat α-amylase [16].The phytic acid also effect the activity of human salivary α-amylase and it was reported that starch digestion has been reduced upto 78% [17].
However, the effect of phytic acid on human salivary α-amylase under different physiological conditions were not thoroughly study yet. In present study, the effect of phytic acid on human salivary amylase activity at different physiological conditions and to interpret the possible mechanism of inhibition by this compound. Since, phytic acid is a common item of diet, especially of vegetarians, discovering of its inhibition of α-amylase would be a significant finding.
Materials and Methods
Extraction of Human salivary α-amylase
About 8-10 ml of mixed saliva was obtained from a healthy person after 2 hours of the breakfast and mouth washed with deionized water. The saliva was centrifuged for 15 minutes at 3000 rpm. The clear supernatant of saliva was separated and stored at –20oC until analyzed [18].
Enzyme assay
The α-amylase activity was estimated by measuring the decrease of absorbance of un-hydrolyzed starch (1.0 mg/ml prepared in phostphate buffer pH 7.0) by colorimetric determination with I2/KI reagent at 620nm using spectrophotometer [19]. 5. 0 ml of substrate was mixed with 0.1ml of 100 fold diluted enzyme and incubated at 37 °C for 10 minutes. After 10 minutes, reaction was stopped by adding 1 ml of 1N HCl.
Effect of substrate concentration
The effect of substrate concentration on the activity of human salivary α-amylase was determined by using various substrate concentrations in enzyme assay. Two ranges of starch concentration were selected for substrate maxima, the low concentration ranges (1mg-6mg/ml) and high concentration ranges (10 mg-60mg/ml).
Effect of phytic acid on the activity of human salivary α-amylase
The effect of phytic acid on the activity of α-amylase was determined by pre-incubation of various concentration of phytic acid ranging from 5 to 40 mM with enzyme prior the addition of substrate for 30 minutes. After that defined amount of enzyme inhibitor mixture was taken for the estimation of enzymatic activity.
Effect of pre-incubation time on the inhibition of α-amylase by phytic acid
The impact of pre-incubation time on inhibition of human salivary α-amylase activity by phytic acid was analyzed through pre-incubation of enzyme with inhibitor for various time intervals ranging from 1-110 minutes.
Effect of reaction pH on the inhibition of human salivary α-amylase by phytic acid
The effect of pH on the inhibition of α-amylase activity by phytic acid in the presence of substrate was determined through the estimation of enzyme activity at different pH levels ranging from (4.0 to 8.0) with the addition of phytic acid with the comparison of absence of phytic acid.
Effect of phytic acid on the kinetic parameters of human salivary α-amylase
The effect of phytic acid on the kinetic parameters (Km and Vmax values) of α-amylase was determined by estimation of enzymatic activity using various concentration of substrate with constant phytic acid concentration.
Statistical analysis
The experimental data was subjected to Graph Pad Prism Software (Version 5.00, 2007) in order to get the values of Km, Vmax and other required statistics. All the experiments were performed in triplicate to get the reliable data.
Results and discussion
Effect of substrate concentration on the activity of α-amylase
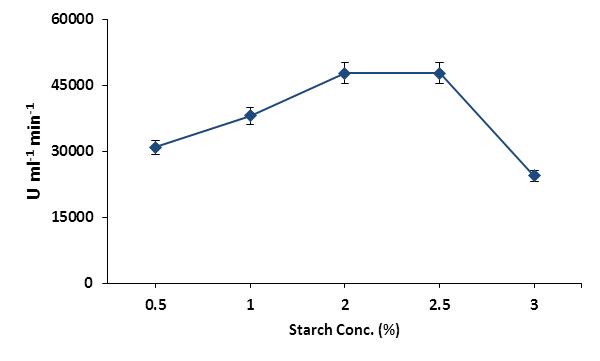
The activities of enzymes are affected by various parameters and the concentration of substrate is a very important parameter to be analyzed for the maximum performance of enzymes. The optimum substrate concentration for maximum catalytic activity of human salivary α-amylase was determined by performing the α-amylase assay in different concentration of starch. The enzymatic activity of α- amylase was increased by increasing the substrate concentration and got at maximum when the starch was reached to 2.0 % (Figure 1). Further increased of starch concentration beyond 2.0 % didn’t influence the enzymatic activity of α-amylase and the enzyme activity remained constant from 2.0 to 2.5 %. The activity of enzyme was reduced by further increased of starch concentration afar 1.0 mg ml-1 and α-amylase lost more than 50 % of its activity at the concentration of 1.5 mg ml-1 of starch. The reduction of enzymatic activity at higher concentration of substrate might be due to formation of greater amount of product and product inhibition of enzyme activity is one of the main regulatory features in metabolism to regulate the enzyme activity through feedback inhibition.
Effect of phytic acid on the activity of α-amylase
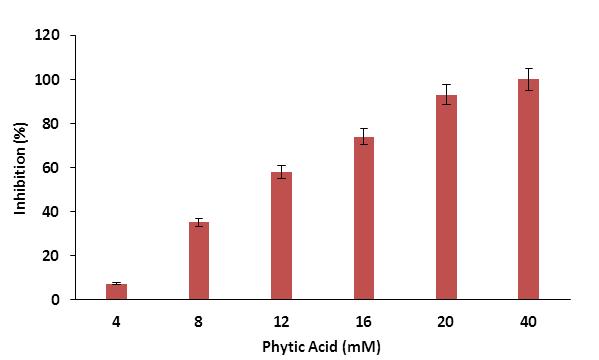
The influence of phytic acid on the activity of human salivary α-amylase was determined and it was observed that the phytic acid significantly affect the activity of amylase (Figure 2). The percent inhibition of amylase activity was increased by increasing the myo-inositol hexaphosphate concentration and human salivary α-amylase lost its complete activity at 40 mM of myo-inositol hexaphosphate. The myo-inositol hexaphosphate is highly negative charged complex carbohydrate with six phosphate groups and strongly binds to the positive charged polar amino acids such as lysine and arginine of protein to modify their structural conformation. This characteristic of myo-inosital hexaphosphate might be responsible for the inhibition of the physiological function of human salivary α-amylase. Similar observation was previously found in case of polyglacturonase inhibition by phytic acid [20].
Effect of pH on the inhibitory activity of phytic acid to human salivary α-amylase
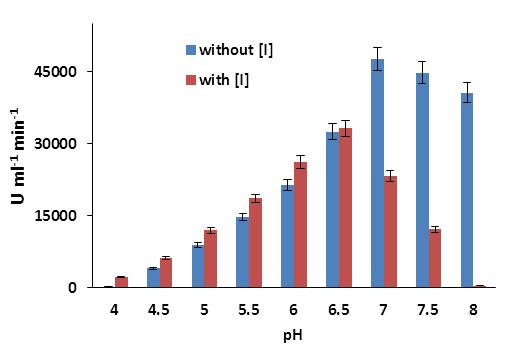
The interaction of phytic acid to other molecules is highly pH-dependent; therefore, the pH-dependent activity of phytic acid on the human salivary α-amylase was analyzed by measuring the enzyme assay in presence of constant concentration of phytic acid (20 mM) at various pH levels with reference to absence of phytic acid. The enzymatic activity of α-amylase significantly changed with pH in the presence of phytic acid (Figure 3). The phytic acid showed positive effect on the activity of human salivary α-amylase in acidic conditions. It enhanced 91%, 34%, 26%, 21%, 19% and 3.0% activity of human α-amylase at pH 4.0, 4.5, 5.0. 5.5, 6.0 and 6.5 as compared to the activity of enzyme on the particular pH in absence of phytic acid, respectively. But, when the acidic range has been ended, the phytic acid turned its behavior and initiated the inhibition of human salivary α-amylase. The phytic acid reduced 52% activity of α-amylase when the pH of the reaction was adjusted to pH-7.0 and 99 % activity of α-amylase was reduced at pH-8.0. The correct protonation state of amino acids on the active site of enzyme is necessary for their catalysis activity. Thus, the pH of the reaction depends on the net pKa value of every amino acids of the enzyme and the alkaline or acidic nature of the substrate. The interaction of phytic acid with amino acids depends on the net charge of amino acids presence on the enzymes, therefore significant changed was observed ons behavior of phytic acids and enzymes with relation of reaction pH. At low pH level, the phytic acid may bind to basic amino acids present in enyme and enforce a comformational change of enzyme structure to increase its catalytic activity. As the pH increased, the intraction behavior of phytic acid towards the amino acids were changed and enforced a negative impact on the conformational structure of enzyme, and reduce its activity as we observed in this study.
Effect of pre-incubation period of enzyme and phytic acid on the activity of human salivary α-amylase
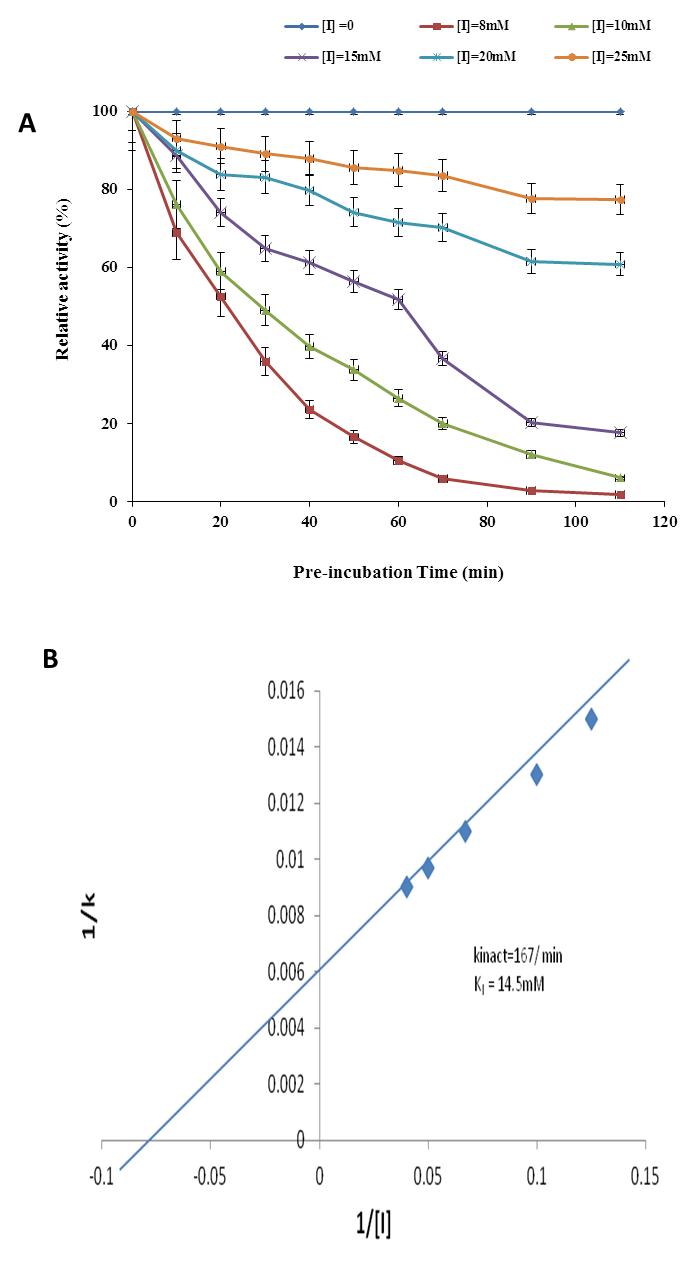
The pre-incubation time of enzyme with various concentration of phytic acid significantly affected the activity of α-amylase (Figure 4A). The activity of enzyme reduced with the increased of pre-incubation time and phytic acid concentration, and maximum inhibition was observed at the period of 90 minutes with 25 mM of phytic acid concentration. An appropriate method for the kinetic analysis of mechanism-based inactivation has been described by Kitz and Wilson [21]. This method is based on the comparative activities of functional enzyme with time following pre-incubation in the presence and absence of inhibitor. The linearity of plot in a declined manner indicates pseudo-first-order inactivation (Figure 4B). The apparent inactivation rate constant (kapp) is described by the slope obtained. In this method, it is assumed that [I] > [El, that EI (the reversible enzyme-inhibitor complex) is at all times in equilibrium with enzyme and inhibitor, and that many reversible enzyme-inhibitor complexes form before one molecule of enzyme is inactivated.
Effect of phytic acid on the kinetic parameters of human salivary α-amylase
The effect of phytic acid on the Km and Vmax values of α-amylase was determined with Lineweaver-Burk plot using GraphPad Prism Software [22]. According to this model, the Km value shows the affinity of enzyme towards its substrate, low Km value means high affinity of enzyme to its substrate and high Km value indicates the low affinity. The experimental value of Km has been increased from 3.945 to 4.945 mM after inhibition, while an apparent decreased in Vmax from 45027 to 28738 U ml-1min-1 has been observed. This could be due to the binding of inhibitor I to both E and ES but unequally that may leads to decreased Vmax and increased Km,app. The resulting plot (Figure 5) shows that inhibition may be mixed non-competitive type because it is effecting both the slope and the y-intercept of Lineweaver-Burk plot. On the basis of the results, the phytic acid can non-competitively inhibit the amylase in different concentration of substrate either at high or low concentration [23-25].
Conclusion
This study is based on the inhibition kinetics of human salivary α-amylase by phytic acid. Phytic acid taken in human diet in an average of 2000-2600mg /day, is a potent inhibitor for the amylases. In the form of sodium phytate salt, complete inhibition of α-amylase occurs at the concentration 40 mM. The inhibition mechanism of phytic acid to human salivary α-amylase was determined through Lineweaver –Burk plot and it was found mixed inhibition. The process is highly pH dependent and a pre-incubation time of 40 minutes favors maximum inhibition at 37 ºC.
Acknowledgements
All authors are thankful to Higher Education Commission, Pakistan for providing financial support for conducting the experimental works.
- Shahrokh Z, Kavoosi G, Shakeri R (2019) Physical, thermal, antioxidant and antimicrobial properties of starches from corn, oat, and wheat enriched with Zataria essential oil. Bioactive Carbohydrates and Dietary Fibre 19: 100193.
- Hizukuri S, Takeda Y, Yasuda M, Suzuki A (1981) Multi-branched nature of amylose and the action of debranching enzymes.Carbohydrate Research 94: 205–13.
- Karimi R, Azizi MH, Sahari MA, Kazem AE (2020) In vitro fermentation profile of soluble dietary fibers obtained by different enzymatic extractions from barley bran. Bioactive Carbohydrates and Dietary Fibre, 21: 100205.
- Gumucio DL, Wiebauer K, Caldwell RM, Samuelson LC, Meisler MH (1988) Concerted evolution of human amylase genes. Mol Cell Biol 8: 1197–205.
- Hamden K, Boujibiha MA, ben Abdeljelil N, Njima M, Achour L (2018) Inhibitory Effect of fermented pectin on key metabolic enzymes associated with diabetes, obesity; and Liver-Kidney tissues toxicities. Bioactive Carbohydrates and Dietary Fibre 16: 82-89.
- Espinal-Ruiz M, Parada-Alfonso F, Restrepo-Sánchez LP, Narváez-Cuenca CE (2014) Inhibition of digestive enzyme activities by pectic polysaccharides in model solutions. Bioactive Carbohydrates and Dietary Fibre 4: 27-38.
- Brune M, Rossander-Hulten L, Hallberg L, Gleerup A, Sandberg AS (1992) Iron absorption from bread in humans: Inhibiting effects of cereal fiber phytate and inositol phosphates with different numbers of phosphate groups. J Nutrition 122: 442–9.
- Hallberg L, Rossander L, Skanberg A (1987) Phytates and the inhibitory effect of bran on iron absorption in man. Ame J Clinical Nutrition 45: 988–96.
- Harland B, Morris E (1995) Phytate: a good or a bad food component? Nutritional Res 15: 733–54.
- McCance R, Widdowson E (1942) Mineral metabolism of healthy adults on white and brown bread dietaries. J Phys 101: 44–85.
- Roberts A, Yudkin J (1960) Dietary phytate as a possible cause of magnesiu deficiency. Nature,185, 823–825.
- Torre M, Rodriguez AR, Saura-Calixto F (1991) Effects of dietary fiber and phytic acid on mineral availability. Cri Rev Food Sci Nutri 1: 1–22.
- Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signaling. Nature 341: 197-205.
- Biswas BB, Biswas S, Chakrabarti S, De BP (1978) A novel metabolic cycle involving myo-inositol phosphates during formation and germination of seeds.In Cyclitols and Phosphoinositides.ed. 57–68.
- Chandra BS, Goel M, Irshad M (1978) Myoinositol hexaphosphate as a potential inhibitor of alpha;-amylases, Phytochemistry 17: 201-4.
- Chandra BS, Goel M, Irshad M (1978) Myoinositol hexaphosphate as a potential inhibitor of alpha;-amylases, Phytochemistry 17: 201-4.
- Knuckles BE, Betschart AA (1987) Effect of phytate and other myo-inositol phosphate esters on α-amylase digestion of starch. J Food Sci 52: 719–21.
- Jawed M, Khan RN, Shahid SM, Azhar A (2012) Clinical study; Protective effects of salivary factors in dental caries in diabetic patients of Pakistan. Experimental Diabetes Res 2012: 1-5.
- Smith BW, Roe JH (1949) A photometric method for the determination of α-amylase in blood and urine, with use of the starch-iodine color. J Biol Chem 179: 53-9.
- Asghar U, Rehman HU, Qader SAU, Maqsood ZT (2013) Influence of phytic acid and its metal complexes on activity of pectin degrading polygalacturonase. Carbohydrate Polymers 95: 167-70.
- Kitz RJ, Wilson IB (1962) Esters of methanesulfonic acid as irreversible inhibitors of Acetylcholinesterase. Journal of the Biological Chemistry. 237: 3245-9.
- Lineweaver H, Burk D (1934) The Determination of Enzyme Dissociation Constants. J Amer Chem Soc 56: 658-66.
- Benjamin WS, Joseph H Roe (1949) A photometric method for the determination of α-amylase in blood and urine, with use of the starch-iodine color. J Biol Chem 179: 53-59.
- Brayer GD, Luo Y, Withers SG (1995) The structure of human pancreatic alpha-amylase at 1.8 A ˚ resolution and comparisons with related enzymes, Protein Science 4: 1730–42.
- Ramasubbu N, Paloth V, Luo Y, Brayer GD, Levine MJ (1966) Structure of human salivary a-amylase at 1.6 A ˚ resolution, Acta Crystalography. D 52: 435-46.

Figures at a glance