Chemical Variability of Essential Oils of Cyperus rotundus from Senegal
Received Date: May 27, 2023 Accepted Date: June 27, 2023 Published Date: June 30, 2023
doi: 10.17303/jocs.2023.1.104
Citation: Yoro Tine, Alioune Diallo, Cheikhouna Gaye, Idrissa Ndoye, Adama Diedhiou et al. (2023) Chemical Variability of Essential Oils of cyperus rotundus from Senegal. J Org Chem Chem Sci 1: 1-11
Abstract
The composition of 30 samples of essential oil isolated from rhizomes of wild-growing Cyperus rotundus harvested in five locations in Senegal was investigated by GC-FID and GC/MS. Essential oils consisted mainly of sesquiterpenes, humulene epoxide 2 (7.0-38.3%), caryophyllene oxide (5.5-28.1%), longiverbenone (3.0-9.4%), cyperotundone (1.2-15.4%), cyperene (0.7-11.1%) and mustakone (1.0-9.1%) being the main components. The compositions of the 30 oils were subjected to kmeans partitioning and principal component analysis, which distinguished two groups within the oil samples. The first group encompassed samples collected Ngano and Ziguinchor. The dominant compounds in this group were of humulene epoxide 2, caryophyllene oxide, mustakone and cyperotundone. The second includes specimens collected from Mont-Rollant (M), Ndiayene Pendao (N) and Guede (G), which have a high percentage of caryophyllene oxide and humulene epoxide 2. The second group is distinguished from the first by significantly higher contents of humulene epoxide 2, caryophyllene oxide and, at the same time, lower contents of mustakone and cyperotundone. To our knowledge, the first chemo type has never been described in the literature.
Keywords: Cyperus rotundus; Rhizome; Essential Oils; GC-MS; Chemical Variability
Introduction
Cyperus rotundus is a member of Cyperaceae family widely distributed in tropical, subtropical, and temperate regions of the world. This plant grows well in almost any soil type, over a wide range of soil humidity, pH, and altitude.It is annual plant that produces prominent swollen underground tuberous bases that remain dormant after the growing season and under adverse conditions [1-3]. Cyperus rotundus is a versatile plant, widely used in traditional medicine around the world and as raw material for perfumes [4-7]. C. rotundus rhizome essential oil possessed antioxidant [6,8,9], antibacterial [6,8-14], insecticide [2,15,16],antimutagenic [6,17], anti-inflammatory [7], antiarthritic [7], analgesic [7] and anticonvulsant activities [7].
C. rotundus tubers show great variation in essential oil composition and several chemotypes have been described.Cyperene (19.2-30.9%) and α-cyperone (4.5-25.2%) were the most abundant constituents in the oils of species from Nigeria and Tunisia [17,18]. Chinese, Iranian and Turkish authors have also reported this high content of cyperene. In China, the essential oil was dominated bycyperene (41.03%), β-caryophyllene (5.32%), α-selinene (4.37%) and α-copaene (4.36%)[19]. In Iran the chemotype was cyperene (16.9%), caryophyllene oxide (8.9%), α-longipinan (8.4%) and β-selinene (6.6%) [20]. In Turkey, cyperene(30.5% and 28.0%), α-copaene (10.6% and 12%) and α-ylangene (7.7% and 10.5%) were identified as the main volatile components of rhizomes [21]. Another study conducted in Iran revealed the presence of elemenone (13.59%), α-cyperone (13.14%), and caryophyllene oxide (13.03%) [15]. The Brazilian species contained α-cyperone (22.8%) and cyperotundone (12.1%) as the main in the essential oil compounds [22]. α-Cyperone (21.1%) has also been reported as the main compound of essential oil from Saudi Arabia along with 4-oxo-α-ylangene (12.8%) [2]. Cyperotundone was also present in the essential oil from Iraq which was mainly composed of two compounds namely cyperene (37.9%) and cyperotundone (11.2%) [23]. The rhizome oils of C. rotundus from India consisted mainly of α-copaene (11.4-12.1%), cyperene (8.04-11.07%), valerenal (8.07-9.08%) and caryophyllene oxide (7.08-9.07%) [24]. Sonwa and Koenig (2001) studied the essential oil of C. rotundus from Germany and found that it was dominated by cyprotene, α-copaene, cyperene,α-selinene and rotundene [25]. More recently, Cisse etal (2021) reported this humulene epoxide 2 (26.1%), caryophyllene oxide (19.2%), longiverbenone (11.3%) chemotype in the essential oil of C. rotundus collected in Senegal [26].
The purpose of this study was to determine the chemical composition of C. rotundus oils collected in different areas in Senegal and to compare this with those of essential oils obtained from other geographical region.
Material and Methods
Plant material
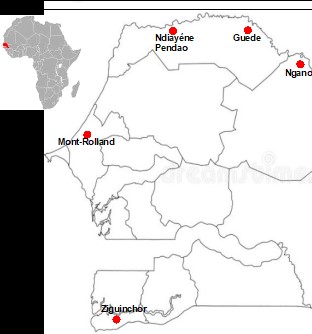
Thirty C. rotundus rhizome samples were collected from five localities in Senegal given in Figure 1: Mont-Rolland (14°55.5988’N, 16°58.59879’W; six samples: M1-M6), Ndiayene Pendao (16°30.24223’N, 15°3.17579’W; seven samples: N1-N7), Guede (16°32.54537’N, 14°45.15816’W; six samples: G1-G6), Ngano (15°16.5628’N,13°2’.52007’W; five samples: NG1-NG5) and Ziguinchor (12°34’336’N, 16°17’35879’W; six samples: Z1-Z6).Each rhizome sample is taken from an area of approximately 200m2. The sampling areas are at least 500 meters apart. The plant material was identified by the technicians from the Botany department of the Fundamental Institute of Black Africa (IFAN) at Cheikh Anta Diop University in Dakar.
Extraction of Essential Oils
Plant samples were air dried for a period of four weeks at room temperature. The plant material was powdered with an average particle size of 0.2 mm using a blade grinder (Polymix PX-MFC 90D, KINEMATICA AG, Luzern,Switzerland). The samples were hydrodistilled (6 h) using a Clevenger-type apparatus according to the method recommended in the European Pharmacopoeia [27].
Chemical Compositions
The chromatographic analyses were carried out using a Perkin-Elmer Autosystem XL GC apparatus (Walthon, MA, USA) equipped with dual flame ionization detection (FID) system and fused-silica capillary columns, namely, Rtx-1 (polydimethylsiloxane) and Rtx-wax (poly- ethyleneglycol) (60 m × 0.22 mm i.d; film thickness 0.25μm). The oven temperature was programmed from 60 to 230°C at 2°C/min and then held isothermally at 230°C for 35 min: hydrogen was employed as carrier gas (1mL/min).The injector and detector temperatures were maintained at 280°C, and samples were injected (0.2 μL of pure oil) in split mode (1:50). The retention indices (RI) of the compounds were determined relative to the retention times of a series of n-alkanes (C5–C30) by linear interpolation using the equation of Van den Dool and Kratz (1963) with the aid of the Perkin-Elmer software (Total Chrom navigator). The relative percentages of the oil constituents were calculated from the GC peak areas, without applying of correction factors.
The samples were also analysed with a Perkin-Elmer Turbo mass detector (quadrupole) coupled to a Perkin-ElmerAutosystem XL, equipped with fused-silica capillary columns Rtx-1 and Rtx-Wax. The oven temperature was programmed from 60 to 230°C at 2°C/min and then held isothermally at 230°C (35 min): hydrogen was used as carrier gas (1 mL/min). The following chromatographic conditions were employed: injection volume, 0.2 μL of pure oil; injector temperature, 280°C; split, 1:80; ion source temperature, 150°C; ionization energy, 70 eV; MS (EI) acquired over the mass range, 35–350 Da; scan rate, 1 s.Identification of the components was based on: (a) comparison of their GC retention indices (RI) on non-polar and polar columns, determined from the retention times of a series of n-alkanes with linear interpolation, with those of authentic compounds or literature data; (b) on computer matching with commercial mass spectral libraries [28-30] and comparison of spectra with those of our personal library; and (c) comparison of RI and MS spectral data of authentic compounds or literature data.
Statistical Analysis
Data analyses were performed using PCA and CA.Both methods aim at reducing the multivariate space in which objects (oil samples) are distributed, but are complementary in their ability to present results. Indeed, PCA provides the data for plots in which both objects (oil samples) and variables (oil components) are represented, while cluster analysis (CA) informs a classification tree in which objects (sample locations) are gathered. The PCA was carried out using the function PCA of the statistical XLSTAT software (Addinsoft, Paris). The discriminate variables (volatile components) have been selected using function of the statistical software. A dendrogram was produced by CA using Ward’s method of hierarchical clustering, based on Euclidean distances between pairs of oil samples.
Results and Discussion
Yields of essential oils given in Table 1 were calculated on the basis of the mass of dry plant matter. They are between 0.6 and 2.6% (Mean ± SD: 1.19 ± 0.53%). These results are consistent with literature reports indicating yields of 0.20–2.60% [2,6,8-11,14,16,18-20,23,31,32].
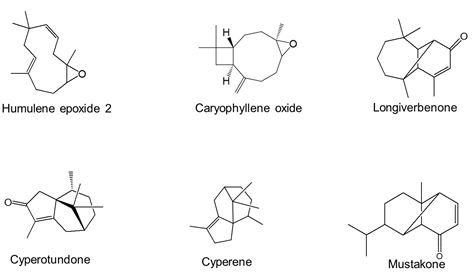
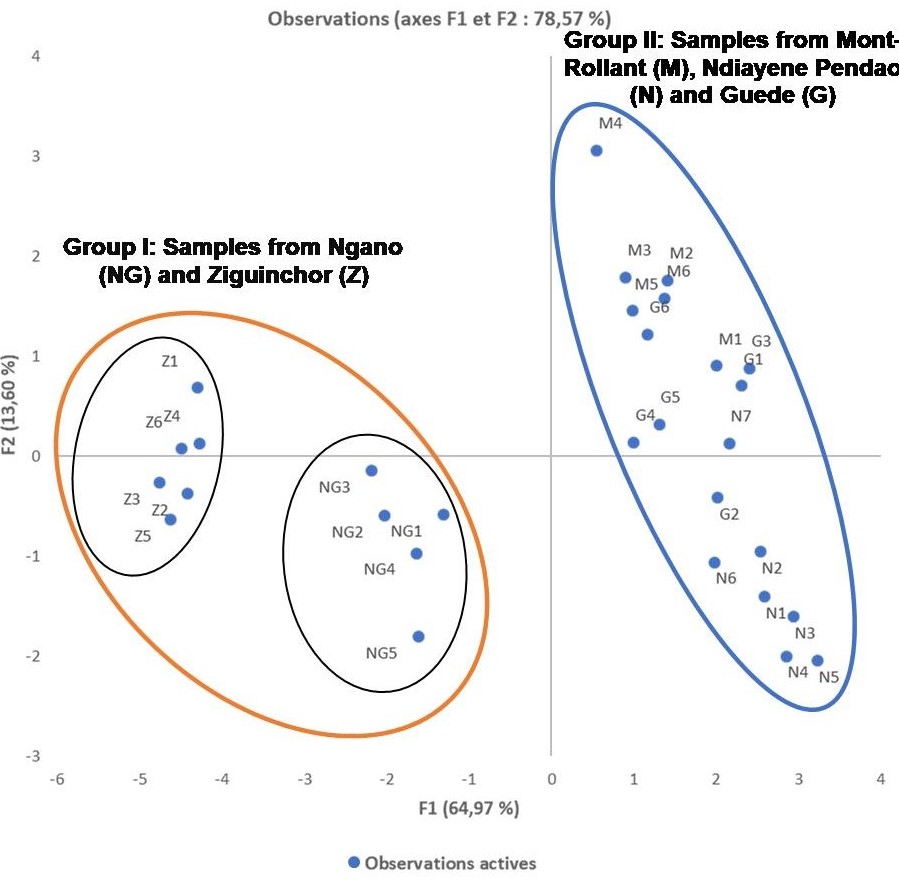
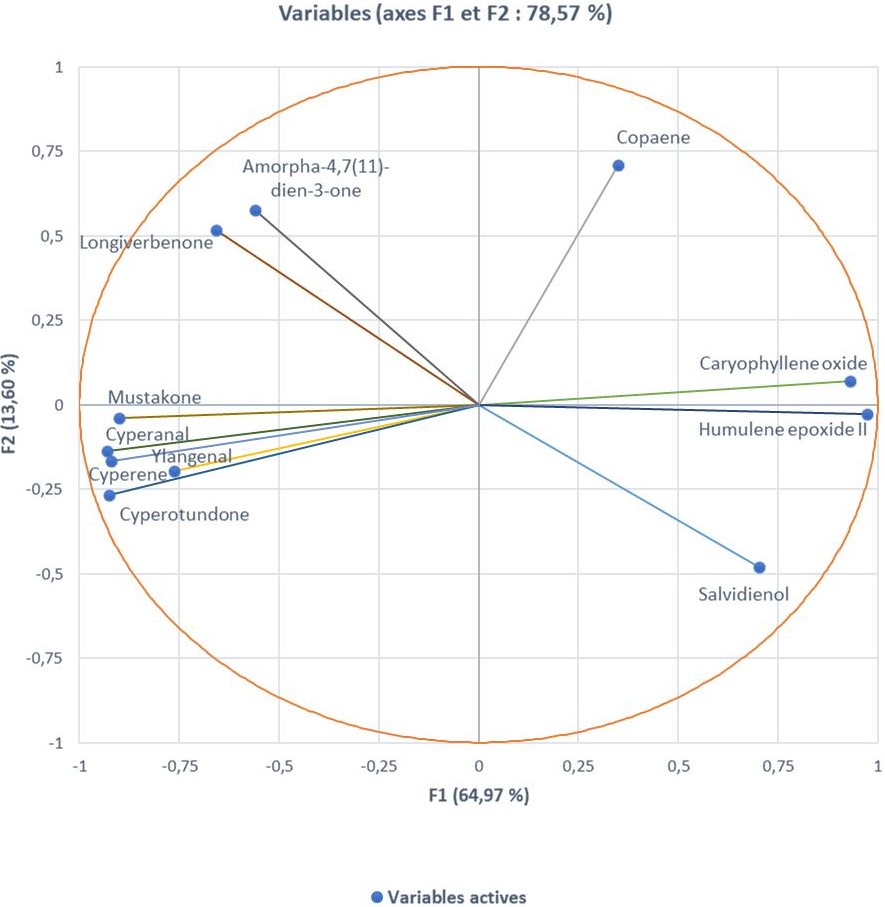
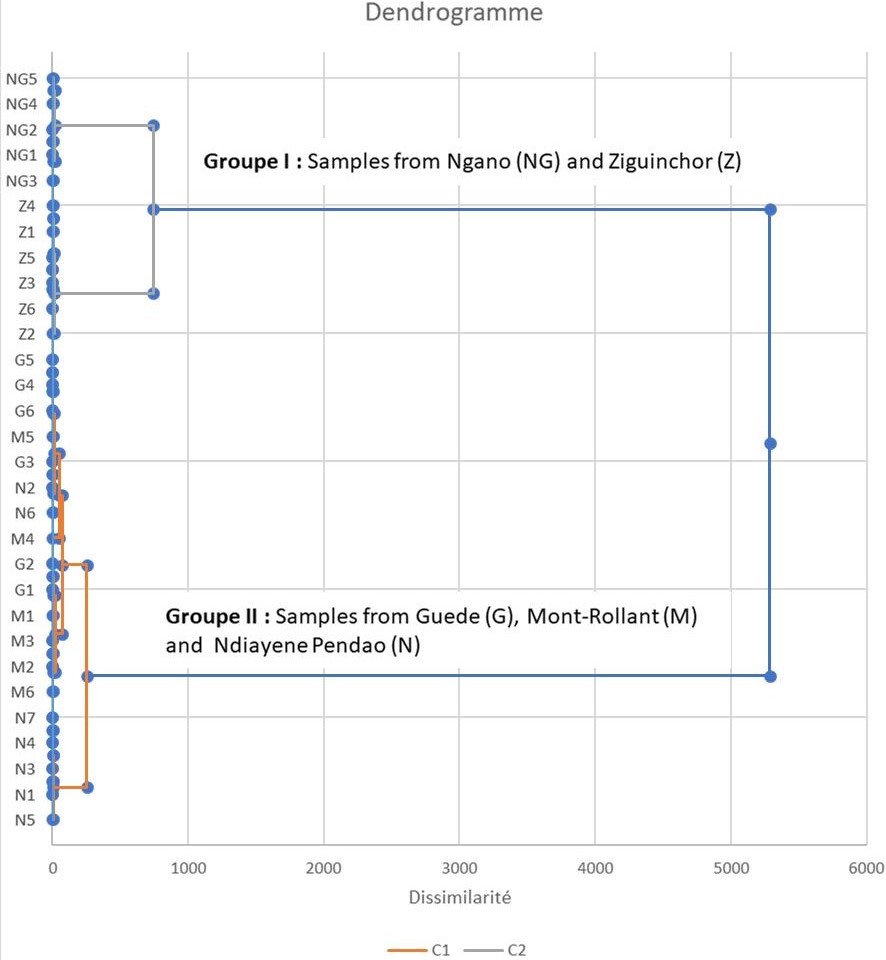
The analysis of the rhizome essential oils by GC/-FID and GC/MS allowed the identification of 49 compounds accounting for 81.0 to 94.0% of the total compositions (Table 1). These essential oils mainly consisted of high amounts of sesquiterpenes. However, the main compounds varied considerably from one sample to another: humulene epoxide 2 (7.0-38.3%), caryophyllene oxide (5.5-28.1%), longiverbenone (3.0-9.4%), cyperotundone (1.2-15.4%), cyperene (0.7-11.1%) and mustakone (1.0-9.1%) (Figure 2). Thus, statistical analyses were performed on the 30 oil compositions to highlight the chemical variability. Figure 3 was obtained from the correlation matrix calculated with the standardized matrix. As shown in Figure 3, the principal factorial plane (constructed with axes 1 and 2) summarizes 78.57% of the entire variability of essential oils. The distribution of oil samples from C. rotundus oils is reported in Figure3a. The distribution of variables (volatile components) is shown in Figure 3b (11 variables). The graph drawn using PCA suggested the existence of two main groups of essential oils (Figure 3a). Statistical analysis using CA was also used to show the correlation between the chemotypes and the geographical distribution of samples. The dendrogram obtained by cluster analysis (CA) reinforced the clustering observed using PCA by grouping the 30 oil samples from C. articulatus into the same two main clusters (Figure 4).
The first group included the samples collected in Ngano (NG) and Ziguinchor (Z). The dominant compounds in this group were of humulene epoxide 2, caryophyllene oxide, mustakone and cyperotundone. This group was divided into two subgroups. The first subgroup, including Ngano samples, showed a high content of humulene epoxide 2 (21.9-23.5%, mean: 22.6%, SD: 0.6%) and cyperotundone(10.2-14.0%, mean: 11.5%, SD: 1.5%). Instead, the high content of mustakone and cyperotundone was a feature of the second subgroup, which included Ngano samples.To our knowledge, these chemotypes have never been described in the literature. However, the presence of mustakone and cyperotundone at significant levels has been reported in a study conducted in Tunisia [8,17]. Cyperotundone has also been described in essential oils of C. rotundus in Asia [32], Iran [20], Marocco [12] and Brazil [22].
The second group includes specimens collected from Mont-Rollant (M), Ndiayene Pendao (N) and Guede (G), which have a high percentage of caryophyllene oxide (18.0-28.1%, mean: 23.2%, SD: 2.9%), humulene epoxide 2(26.4-38.3, mean: 32.4%, SD: 3.3%). Similarly, high contents of humulene epoxide 2 and caryophyllene oxide have been reported for C. rotundus oil from Louga region in Senegal.However, these compounds were at low levels or absent in essential oils of C. rotundus from Iran [20], India [24] and Brazil [24].
In summary, the results obtained from the analysis of the thirty samples have led to the establishment of three chemotypes to C. rotundus essential oils. These results are based on single samples to each collection site and do not account for within site variation. However, the chemical composition of the analyzed essential oils showed qualitative and quantitative variation by the influence of the localization.
Conclusion
The present investigation demonstrated the important of chemical variability of the essential oils isolated from the rhizomes of C. rotundus populations growing wild in Senegal. Principal component analysis (PCA) and cluster analysis (CA) (dendrogram) applied on the matrix linking essential oil compositions and sample locations allowed the distinction of two groups within the oil samples. The dominant compounds in the first group were humulene epoxide2, caryophyllene oxide, mustakone and cyperotundone and those in the second group were caryophyllene oxide and humulene epoxide 2. To our knowledge, we have reported the first chemotype for the first time. In perspective, we will evaluate their biological activities and also produce cosmetic formulations based on these essential oils.
- Eltayeb IM, ELAmin AM, Elhassan I, Ayoub SMH (2016) A Comparative Study of the Chemical Composition of Essential Oils of Cyperus Rotundus L.(Cyperaceae) Growing in Sudan. American Journal of Research Communication 4:28-72.
- Al Massarani, S Al-Enzi, F Al-Tamimi, M Al-Jomaiah,N Al-amri et al. (2016) Composition & Biological Activity of Cyperus Rotundus L. Tuber Volatiles from Saudi Arabia.Natural Volatiles and Essential Oils 3: 26-34.
- Umerie SC, Ezeuzo HO (2000) Physicochemical Characterization and Utilization of Cyperus Rotundus Starch.Bioresource Technology 72: 193-6,
- Al-Snafi AE (2016) A Review on Cyperus Rotundus A Potential Medicinal Plant. IOSR Journal of Pharmacy 6:32-48.
- Jeurkar M, Kosalge S, Sheikh D, Telrandhe U (2022) Cyperus Rotundus L Phytochemistry and Pharmacological Activities. Annals of Phytomedicine: An International Journal 11: 186-96.
- Hu QP, Cao XM, Hao DL, Zhang LL (2017) Chemical Composition, Antioxidant, DNA Damage Protective, Cytotoxic and Antibacterial Activities of Cyperus Rotundus Rhizomes Essential Oil against Foodborne Pathogens. Sci Rep 7:45231.
- Biradar S, Kangralkar VA, Mandavkar Y, Thakur M,Chougule N (2010) Antiinflammatory, Antiarthritic, Analgesic and Anticonvulsant Activity of Cyperus Essential Oils.Int J Pharm Pharm Sci 2: 112-15.
- Essaidi I, Koubaier HBH, Snoussi A, Casabianca H,Chaabouni MM (2014) Bouzouita, N. Chemical Composition of Cyperus Rotundus L. Tubers Essential Oil from the South of Tunisia, Antioxidant Potentiality and Antibacterial Activity against Foodborne Pathogens. Journal of Essential Oil Bearing Plants 17: 522-32,
- Kilani S, Ledauphin J, Bouhlel I, Sghaier MB, Boubaker J et al. (2008) Comparative Study of Cyperus Rotundus Essential Oil by a Modified GC/MS Analysis Method. Evaluation of Its Antioxidant, Cytotoxic, and Apoptotic Effects.Chemistry & Biodiversity 5: 729-42.
- Yagi S, Babiker R, Tzanova T, Schohn H (2016) Chemical Composition, Antiproliferative, Antioxidant and Antibacterial Activities of Essential Oils from Aromatic Plants Growing in Sudan. Asian Pacific journal of tropical medicine 9: 763-70.
- Zhang LL, Zhang LF, Hu QP, Hao DL, Xu JG (2017) Chemical Composition, Antibacterial Activity of Cyperus Rotundus Rhizomes Essential Oil against Staphylococcus Aureus via Membrane Disruption and Apoptosis Pathway. Food Control 80: 290-6.
- Siroua K, El Ghallab, Y Aït Mouss, R Kadiri, F Belamine, H El Kouali, M Kenz (2022) A Chemical Composition of Essential Oil from Invasive Moroccan Cyperus Rotundus L., in Vitro Antimicrobial and Antiradical Activities, and in Silico Molecular Docking of Major Compounds on Drug Efflux Pumps. South African Journal of Botany 147: 782-9.
- Mojab F, Vahidi H, Nickavar B, Kamali-Nejad M (2009) Chemical components of essential oil and antimicrobial effects of rhizomes from Cyperus rotundus L. Journal of Medicinal Plants 8: 91-186.
- Singh V, Ali M, Negi A, Sultana S (2018) Analysis and Antimicrobial Activity of the Essential Oil of Cyperus Rotundus L. Rhizomes. J Med Plants Stud 6: 101-5.
- Janaki S, Zandi-Sohani N, Ramezani L, Szumny A (2018) Chemical Composition and Insecticidal Efficacy of Cyperus Rotundus Essential Oil against Three Stored Product Pests. International Biodeterioration & Biodegradation 133: 93-8.
- Liu XC, Lu XN, Liu QZ, Liu ZL (2016) Chemical Composition and Insecticidal Activity of the Essential Oil of Cyperus Rotundus Rhizomes Against Liposcelis Bostrychophila (Psocoptera: Liposcelididae). Journal of Essential Oil Bearing Plants 19: 640-7.
- Kilani S, Abdelwahed A, Ammar RB, Hayder N,Ghedira K et al. (2005) Chemical Composition, Antibacterial and Antimutagenic Activities of Essential Oil from (Tunisian) Cyperus Rotundus. Journal of Essential Oil Research 17: 695-700.
- Ekundayo O, Oderinde R, Ogundeyin M, Stahl- Biskup E (1991) Essential Oil Constituents of Cyperus Tuberosus Rottb. Rhizomes. Flavour and Fragrance Journal 6: 261-4.
- Chen Y, Zhao YY, Wang XY, Liu JT, Huang LQ et al.(2011) [GC-MS analysis and analgesic activity of essential oil from fresh rhizoma of Cyperus rotundus]. Zhong Yao Cai 34:1225-9.
- Ghannadi A, Rabbani M, Ghaemmaghami L, Malekian N (2012) Phytochemical Screening and Essential Oil Analysis of One of the Persian Sedges; Cyperus Rotundus L. International Journal of Pharmaceutical Sciences and Research 3: 424.
- Poyraz İE, Demirci B, Küçük S (2018) Volatiles of Turkish Cyperus Rotundus L. Roots. Records of Natural Products 12.
- Zoghbi M, das G, Andrade EH, Carreira LM, Rocha EA (2008) Comparison of the Main Components of the Essential Oils of “Priprioca”: Cyperus Articulatus Var. Articulatus L, C Articulatus Var. Nodosus L, C Prolixus Kunth and C. Rotundus L. Journal of Essential Oil Research 20: 42-5.
- Aghassi A, Naeemy A, Feizbakhsh A (2013) Chemical Composition of the Essential Oil of Cyperus Rotundus L.from Iran. Journal of Essential Oil Bearing Plants 16: 382-6.
- Jirovetz L, Wobus A, Buchbauer G, Shafi MP,Thampi PT (2004) Comparative Analysis of the Essential Oil and SPME-Headspace Aroma Compounds of Cyperus Rotundus L. Roots/Tubers from South-India Using GC, GC-MS and Olfactometry. Journal of Essential Oil Bearing Plants 7:100-6.
- Sonwa MM, König WA (2001) Chemical Study of the Essential Oil of Cyperus Rotundus. Phytochemistry 58:799-810.
- Cisse K, Gassama D, Thiam A, Ndiaye EB, Gueye MT, Fall M (2021) Comparative Study of S235 Steel Corrosion Inhibition by Eucalyptus Camaldulensis and Cyperus Rotundus Essential Oils in Hydrochloric Acid Solution. American Journal of Physical Chemistry 10: 6-15.
- Council of Europe (1997) European Pharmacopoeia.;3rd ed.; Council of Europe: Strasbourg, ISBN 978-92-871-2991-8.
- Joulain D, König WA (1998) The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; EB-Verlag.
- Adams RP (2007) others Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry.;Allured publishing corporation.
- NIST (National Institute of Standards and Technology) (2016) N PC Version of the NIST/EPA/NIH Mass Spectra Library
- Abo-Altemen RA, Al-Shammari AM, Shawkat MS (2019) GC-MS Analysis and Chemical Composition Identification of Cyperus Rotundus L. from Iraq. Energy Procedia 157: 1462-74.
- Lawal OA, Oyedeji AO (2009) Chemical Composition of the Essential Oils of Cyperus Rotundus L. from South Africa. Molecules 14: 2909-17.

Tables at a glance
Figures at a glance