Comparison of Adenosine deaminase (ADA) and Antigen 125 (CA-125) level in Patients with Colon Cancer and Healthy Control
Received Date: July 01, 2023 Accepted Date: August 01, 2023 Published Date: August 04, 2023
doi: 10.17303/jocs.2023.1.105
Citation: Ebrahimi-Rad M, Khatami Sh, Chigini H, Kariman A, Akhbari H et al. (2023) Comparison of Adenosine deaminase(ADA) and Antigen 125 (CA-125) level in Patients with Colon Cancer and Healthy Control. J Org Chem Chem Sci 1: 1-9.
Abstract
Aim: Colorectal cancer is the third common cancer in the world. This cancer develops slowly and silently so it is usually detected very late. Finding some simple methods for early diagnosis can lead to timely prevention and treatment of the cancer. Molecular biomarkers are factors that assessing their roles in diagnosis, prognosis and follow-up different diseases especially cancers have got a lot of attention in recent years. CA-125 and ADA are two proteins that their increase has been observed in various cancers. In this study we want to compare ADA and CA-125 level in colorectal cancer patients and healthy controls.
Methods: 50 blood samples from patients and 50 samples from healthy control were obtained and ADA and CA-125 were assessed in both groups. ADA level was assessed using enzymatic method by autoanalyzer (BT3000). CA-125 was measured by Elecsis based on Chemiluminescence. Data was analyzed by SPSS 16 software.
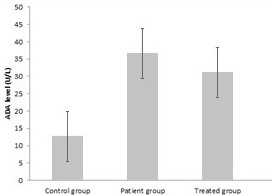
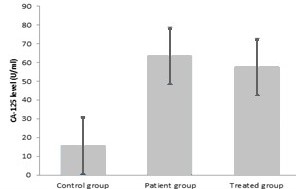
Results: The mean level of ADA in patients group was 36.57 ± 1.5 U/L. Its level was 12.83±5.7 U/L in control group. The average value of CA-125 in patients and healthy control were 63.54 U/ml and 15.67 U/ml, respectively.
Conclusion: Based on obtained results, ADA level was increased significantly compared to control group. (P<0.05) Although serum CA-125 level showed no notable differences.
Keywords: Colorectal cancer; Colon cancer; Cancer antigen 125; Adenosine deaminase; ADA; CA-125
Introduction
Colorectal cancer (CRC) is a very common cancer in many countries [1]. CRC is an asymptomatic disease at the beginning and develops slowly so it can be treated easily but it causes death in most of the patients due to the late diagnosis [2,3]. There are some known agents that are related to the incidence of this cancer that among them family history, age, host microbes and some mutable factors like diet, obesity and low physical activity are important ones that have been studied [4]. Age is the most important factor such that the caner prevalence is 99% in people older than 40 and 85% in people older than 60 [5]. Most of the patients refer to doctors with pain in abdomen, anaemia, loss of weight and digestive changes but some of them even don’t have these manifestations and their disease is known after metastasis [3,6]. CRC can be prevented if it has been diagnosed on time [3]. Routine screening methods for diagnosing and evaluating CRC are fecal occult blood testing, colonoscopy, computed tomographic colonography and flexible sigmoidoscopy. Most of these tests are expensive and invasive, so finding some other ways for screening CRC seems to be essential. Molecular biomarkers are some potential agents that can be measured as primary screening markers. They can be used for follow-up people who have received treatment [7]. They can help physicians to diagnose the caner and prevent deaths caused by colorectal cancer because of on time treatment [3], but lack of suitable markers for diagnosing cancer are always felt [8]. Carcinoembryonic antigen (CEA) is a tumor marker that has been assessed a lot and it is approved as a good marker for screening colorectal cancer specially for follow-up because of its high specificity and sensitivity but it is not applicable alone. This issue can be solved by finding some other useful markers [9].
Cancer antigen 125 (CA 125) is a glycoprotein tumor marker [10,11] which exists in the surface of some tissues including lung, endometrium and fallopian tube [12]. It increases in some pathological conditions like ovarian cancer and some other malignancies such as cervix and lung cancer but its elevation can be observed in some normal conditions, too [11,13]. This marker is mostly used in screening the ovarian cancer but because of low specificity and sensitivity in detection of early stage of the disease, it is better to combine it with other markers [12].
Adenosine deaminase (ADA) is an important player in purine metabolism [14]. This enzyme converts adenosine to inosine and deoxyadenosine to deoxyinosine by deaminating them [15]. ADA exists in different human tissues and its metabolites contribute in different processes like proliferation and differentiation of various cells including T cells [16]. So the ADA activity in diverse diseases like some cancers has been considered in some researches [15,17]. The activity of ADA is elevated in some diseases such as leukemia, maliganancy, brucellosis, acute pneumonia, rheumatoid arthritis and breast cancer which shows the cellular system stimulation [15,18]. Therefore measuring the level of ADA may be useful in diagnosing of some diseases.
In this study we evaluate the level of ADA and CA-125 in serum of patient who is diagnosed with colorectal cancer.
Methods
Patients
In this research, healthy individuals and patients diagnosed with colorectal cancer who were hospitalized in Loghman hospital and different parts of cancer institute of Iran including men cancer, women cancer and intermedical oncology were participated. The study was done from February 2014 to August 2016 with respect to medical ethics and consent of participants.
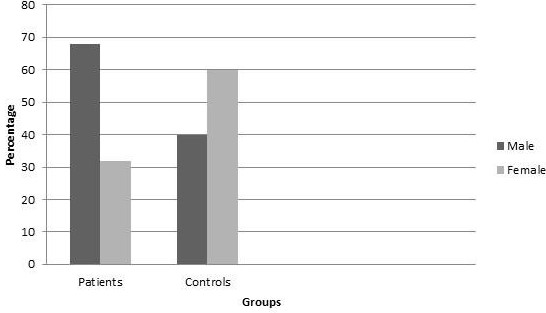
50 specimens were collected from healthy controls and patients separately. The identical factors were measured in both groups. 68% participants were men and 32% were women. Most patients were in age 46 to 55.
Assays
3 ml of blood sample was obtained from each participants. The specimens stayed at room temperature for 30 minutes and after clotting, they were centrifuged for 5 minutes at 2500 rpm, then the above layer which was serum were collected and kept at -70°C for measuring ADA and CA-125.
ADA was assayed by Zist shimi kit and BT3000 autoanalyzer based on photometric method. ADA normal range is less than 40 U/L. Kit calibrator was used to calibrate the apparatus. For setting the considered test on apparatus ADA control which exist in the kit was used. The absorbance was measured in 340 nm.
CA-125 antigen was assayed using chemiluminescence technique and ECL Elecsis immunoanalyser. Its normal range is 0 to 35 U/ml.
Statistical analysis
Data was showed as mean ± SD. Analysing data was done by SPSS 16 with student t-test method. The P value less than 0.05 was considered significant.
Results
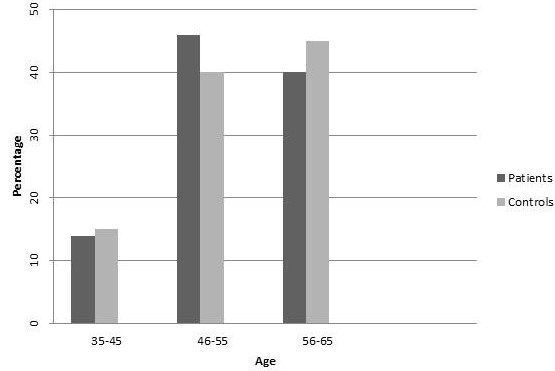
The patients in this study were in following age range: 14% (35 to 45), 46% (46 to 55) and 40% (56 to 65) years old. (Figure 1) 32% of patients were women and 68% were men (Figure 2) 40% of healthy group were male and 60% were female. The percentage of participants in each age range was: 15% (35-45) , 40% (46-55) and 45% (56-65) in healthy controls. The data was obtained by comparing control group with test group.
The mean level of ADA in patient group was 36.57 ± 1.5 U/L .It has a mean level of 12.83 ± 5.7 U/L in control group.(Table 1 and fig 3) These findings showed that the ADA level was much higher in patient group compared with reference group , so their difference was statistically significant.
The average value of CA-125 in patients and healthy control were 63.54 U/ml and 15.67 U/ml respectively. (Table 2 and figure 4) Although CA-125 value was increased in patient group, the comparison of CA- 125 value in these two groups did not show any significant differences.
ADA and CA-125 level were also assayed in serum of 12 patients receiving treatment. The mean ADA level was 31 U/L and CA-125 was 57.65 U/ml after treatment.These amounts show a little decrease in ADA and CA- 125 level after treatment.
Discussion
Despite many advances in prevention and diagnosis of colorectal cancer, many people die from this cancer annually. OBT is the primary test for screening colorectal cancer patients but it is not sufficient alone because of low sensitivity. Endoscopy, colonoscopy and biopsy are the gold standard tests for precise diagnosis [6]. Most of the methods available to screen colorectal cancer are either invasive or unpleasant to patients, so this leads to fewer patients being screened for the disease. Discovering the cancer before development or even before appearing at the level of polyps helps physicians to apply useful treatments and prevent the disease occurrence or spreading, so it can cause more survival rate. Finding some markers that can help in early and easy diagnosis has always been in the center of attention. Recently using molecular biomarkers for diagnosis and screening different cancers is becoming more common because of their easy accessibility. These markers should be sensitive, specific and simply measurable, so they can be reliable to be used for early diagnosis, monitoring the disease and prognosis [3]. Increase in ADA level as a molecular biomarker has been reported in different cancers. We measured ADA level in colorectal cancer patients in our study. A significant elevation in serum ADA activity and a decrease after treatment was observed according to our study. An explanation for this increase is the elevated turnover of DNA in cancerous tissues that causes increment in ADA as an metabolizing enzyme of adenosine [19]. These results are in agreement with other studies. In the primary studies on correlation between ADA and cancers, in 1957, Straub et al showed that ADA level was increased in 91% of 527 cancer patients [20]. In a study that was done by Joop ten and colleagues in 1984, an average increase of 40% in ADA was observed in tumors of colorectal adenomatous patients compared to control tissues [21]. In 1972, Dzieza-Lalowicz and Hankiewicz demonstrated that ADA activity in gastric juice of gastric cancer patients is significantly high [22]. Since adenosine, the ADA substrate, seems to be toxic for cells, a hypothesis for ADA elevation in cancers represents that increase in ADA level may be due to neutralizing this toxic effect and helping tumor growth [23]. As a controversial study Kojima et al showed a decrease in ADA level in peripheral blood lymphocyte of colorectal cancer patients which might be because of immune suppression in cancer patients [24].
We also measured CA-125 in the serum of participants. Although CA-125 level was increased in 40% of patients compared to control group but it was not statically significant. Increase in the serum level of CA-125 has been reported in other studies especially on ovarian cancer but it is not considered as a sensitive and specific maker for early diagnosis. CA-125 value is usually elevated in 85% of ovarian cancer cases [10]. Scientists have combined CA-125 with other markers to improve its sensitivity. In a study which was done by Bashizadeh- Fakhar et al, determining combination of CA-125 with CEA and ROMA indicated a promising biomarker for ovarian cancer detection [11]. Streppel et al showed that CA-125 is also expressed in colorectal cancer patients. It is considered that CA-125 interaction with different receptors causes cancer cells to escape from apoptosis.
Connection of CA-125 to GPI-anchored glycoprotein helps tumors to implant and metastasize. Also a receptor on immune cells has been investigated which inhibits immune responses. These are some of the mechanisms by which CA-125 can promote cancer growth [25].
In most of the studies that CA-125 level was evaluated in colorectal or other gastrointestinal cancer patients, no significant increase was observed in patients group which is compatible with the result of our study. Although CA-125 status decrease significantly after treatment and could be considered as a treatment monitoring factor beside other markers. For example, in a study with Yamao and colleagues, it was demonstrated that CA-125, CEA and CA-19 decrease after chemotherapy [26]. In 2016, Basu et al also determined some tumor markers in colorectal cancer patients before and after treatment and they reported that elevation in CA-125 level in patients group was not significant compared to controls but the decrease in its level was remarkable after therapy. So this marker along with other markers can be used to assess chemotherapy effectiveness [27]. In a study in 2018 which was done by Gao et al, it was found that among different tumor markers, CA-125 has the lowest sensitivity in colorectal cancer detection and combining that with other markers can improve the sensitivity [28]. So based on the results of these studies and our finding, CA-125 cannot be a useful marker for colorectal cancer on its own.
Although measuring tumor markers can be a useful method for detection and monitoring colorectal cancer but utilizing a panel of appropriate biomarkers can help to achieve more trustworthy results. Further research is needed to clarify the importance of biomarkers in colon cancer and to obtain validated findings.
- Haddad FG, Eid R, Kourie HR et al. (2018) Prognostic and predictive biomarkers in nonmetastatic colorectal cancers. Future Medicine 14: 2097-102.
- Liu SL, Cheung WY (2019) Role of surveillance imaging and endoscopy in colorectal cancer follow-up: Quality over quantity? World journal of gastroenterology 7: 25-59.
- Mahasneh A, Al-Shaheri F, Jamal E (2017) Molecular biomarkers for an early diagnosis, effective treatment and prognosis of colorectal cancer: current updates. Experimental and molecular pathology 102: 475-83.
- Center MM, Jemal A, Smith RA et al. Worldwide variations in colorectal cancer. CA: a cancer journal for clinicians 59: 366-78.
- Winawer S, Fletcher R, Rex D et al. (2003) Colorectal cancer screening and surveillance: clinical guidelines and rationale update based on new evidence. Gastroenterology 124: 544-60.
- Buccafusca G, Ilaria P, Antonino T et al. (2019) Early colorectal cancer: diagnosis, treatment and survivorship care. Critical reviews in oncology/hematology 13: 109-31.
- Gluecker TM, Johnson CD, Harmsen WS et al. (2003) Colorectal cancer screening with CT colonography, colonoscopy, and double-contrast barium enema examination: prospective assessment of patient perceptions and preferences. Radiology 227: 378-84.
- Hori SS, Gambhir SS (2011) Mathematical model identifies blood biomarker–based early cancer detection strategies and limitations. Science translational medicine 3: 109ra116.
- Plebani M, De Paoli M, Basso D et al. (1996) Serum tumor markers in colorectal cancer staging, grading, and follow‐up. Journal of surgical oncology 62: 239-44.
- Perkins GL, Slater ED, Sanders GK et al. (2003) Serum tumor markers. American family physician 68: 1075-88.
- Bashizadeh-Fakhar H, Rezaie-Tavirani M, Zali H et al. (2018) The Diagnostic Value of Serum CEA, CA-125, and ROMA Index in Low-Grade Serous Ovarian Cancer. International Journal of Cancer Management 11: 1-6.
- RC JB, Xu FJ, Yu YH et al. (1998) CA 125: the past and the future. International Journal of Biological Markers 13: 179-87.
- Tuxen MK, Sölétormos G, Dombernowsky P (1995) Tumor markers in the management of patients with ovarian cancer. Cancer treatment reviews 21: 215-45.
- Muraoka T, Katsuramaki T, Shiraishi H et al. (1990) Automated enzymatic measurement of adenosine deaminase isoenzyme activities in serum. Analytical biochemistry 187: 268-72.
- Salmanzadeh S, Soleimani M, Mohammadi MJ et al. (2018) Diagnostic Value of Serum Adenosine Deaminase Level in Extrapulmonary Tuberculosis. Avicenna J Clin Microbiol Infect 5: 77-81.
- Van der Weyden MB, Kelley WN (1976) Human adenosine deaminase. Distribution and properties. Journal of Biological Chemistry 251: 5448-56.
- Pirinççi N, Kaya TY, Kaba M et al. (2017) Serum adenosine deaminase, catalase, and carbonic anhydrase activities in patients with renal cell carcinoma. Redox Report 22: 252-6.
- Kutryb‐Zajac B, Koszalka P, Mierzejewska P et al. (2018) Adenosine deaminase inhibition suppresses progression of 4T1 murine breast cancer by adenosine receptor‐dependent mechanisms. Journal of cellular and molecular medicine 22: 5939-54.
- Suchitra MM, Reddy EP, Sudhakar GM et al. (2009) Evaluation of serum adenosine deaminase as a tumor marker in gastric cancer. Res J Med & Med Sci 4: 411-4.li>
- Schwartz MK, Bodansky O (1959) Serum adenosine deaminase activity in cancer. Proceedings of the Society for Experimental Biology and Medicine 101: 560-2.
- Ten Kate J, Wijnen JT, van der Goes RG et al. (1984) Quantitative changes in adenosine deaminase isoenzymes in human colorectal adenocarcinomas. Cancer research 44: 4688-92.
- Dzieza-Lalowicz I, Haniewicz J (1972) Adenosine deaminase in gastric juice. Enzymologia 42: 23-9.
- Namiot Z, Kemona A, Stasiewicz J et al. (1994) Adenosine deaminase activity in gastric cancer. Cancer letters 82: 95-8.
- Kojima O, Majima T, Uehara Y et al. (1985) Alteration of adenosine deaminase levels in peripheral blood lymphocytes of patients with gastric cancer. The Japanese journal of surgery 15: 130-3.
- Streppel MM, Vincent A, Mukherjee R et al. (2012) Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Human pathology 43: 1755-63.
- Yamao T, Kai S, Kazami A et al. (1999) Tumor markers CEA, CA19-9 and CA125 in monitoring of response to systemic chemotherapy in patients with advanced gastric cancer. Japanese journal of clinical oncology 29: 550-5.
- Basu A, Seth S, Chauhan AK et al. (2016) Comparative study of tumor markers in patients with colorectal carcinoma before and after chemotherapy. Annals of translational medicine 4: 71.
- Huang CJ, Jiang JK, Chang SC et al. (2016) Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine 95: e5177.

Tables at a glance
Figures at a glance