The Use of Modify Chitosan Sorbent to Obtaining of Biopolymer Granules with 131Cs Radionuclide for Brachytherapy
Received Date: January 03, 2025 Accepted Date: February 03, 2025 Published Date: February 06, 2025
doi: 10.17303/jocs.2025.3.101
Citation: E.A. Markelova; S. Khujaev; A. Vasidov (2025) The Use of Modify Chitosan Sorbent to Obtaining of Biopolymer Granules with 131Cs Radionuclide for Brachytherapy. J Org Chem Chem Sci 3: 1-8
Abstract
In this work was described the creation method of the hermetic granules of the X-emitters on base of 131Cs for using in brachytherapy. The chitosan and modified additives are being used as a sorbent for 131CsCl ions. The maximal sorption of 131CsCl ions was obtained by adding to chitosan solution the potassium ferro(II)cyanide and copper or nickel chlorides. For forming the granules of X-emitters, the radioactive drops through of syringe were serially passed through air layer, solutions of the xylene and alkali sodium. The received granules has been crosslinking with glutaraldehyde solution for its forming hardness and hermeticity. The diameters and radioactivity of the granules can be regulated within 0.5±0.05 – 1.0±0.1 mm and (7.4 – 22.2) 107 Bq, respectively.
Keywords: Cesium-131; Chitosan; Modified Additives; Glutaraldehyde; Brachytherapy
Introduction
The chitosan and its derivatives are used widely in nuclear medicine as therapeutic preparations. Chitosan compounds used in the pharmaceutical industry have several advantages because of biodegradable, biocompatible, acceleration of wound healing, reduction of blood cholesterol level and inhibition of tumor cells. The natural polymers chitin and chitosan, belonging to the class of polysaccharides – high-molecular compounds that built from elementary units of links of monosaccharides, that connected by glycosidic bonds. The chemical structure of the chitin is a linear aminopolysaccharide that a consisting of units of N-acetyl-2-amino-2-deoxy-D-glycopyranose. A completely deacetylated product - poly [(1-4) -2-amino-2-deoxy-b-D-glucose] - is called chitosan [1-3]. It is insoluble in aqueous, alkali, organic solvents, while it is soluble in acid solutions when the pH is less than 6.0 [2,3]. There are some scientific works on applications of chitosan derivatives for purification wastes, solutions from hazardous radionuclides. For example, in [4] chitosan benzoyl thiourea derivative was used for the removal of the hazardous 60Co and 152+154Eu radionuclides from aqueous solutions. In the other work [5], chitosan was used as a sorbent after modifying by ferrocyanide additives and second group of transitive metals for purification of the aqueous solutions from 137Cs radionuclides. Scientific data on application of natural biopolymers in nuclear medicine, chiefly in the brachytherapy are very limited. The authors of [6], created the mode of preparation 131I-collagen-chitosan microspheres by using chloramines-T or N- bromosuccinimide for treatment of the lesion organs by injecting 131I-microspheres in tumour area. The method of precipitation β-emitters of 153Sm, 165Dy, 166Ho, 90Y in solid chitosan sorbents for using in radiosynovectomy has been described in [7-9]. At present, the 125I, 103Pd and 131Cs radionuclides in titanium capsules widely used in brachytherapy, as an X-emitters [10,14,15]. These capsules are implanted in tumour area of body, to effectively treat the cancer cells, without damaging nearby healthy tissue and thereby reducing negative side effects. Brachytherapy experts in the American company “IsoRayMedical” (www.isoray.com) have given their prefer like to 131Cs (T1/2=9.8 d, Eγ=31 keV) than to 125I (T1/2=60 d; Eγ=28 keV) and 103Pd (T1/2=17 d; Eγ=21 keV), because of its shorter half-life and higher X- ray energy [10]. Except titanium capsules, the X-ray radionuclides can be encased in a resin [11], glass [12] and an organic beads [13]. Despite of availability of various kinds of the X-ray sources that intended for destruction of malignant tissues, each of one has merits and defects, which connected with cost and technological capability of production. The capsulation of the 131Cs radionuclides in titanium capsules a represents technical and financing difficulties for us. Therefore, are need elaborated and created a relatively simple and cheap method for domestic brachytherapy. In our previous studies were developed the method of obtaining 131CsCl ions under microwave radiation [14] with 99.97% radiochemical purity [15]. Therefore, the aim of this study was elaboration the technique to creation a hermetic, spherical, biopolymer X-emitters on the base 131Cs radionuclides are being with biocompatible and biodegrading properties for brachytherapy.
Experimental Procedure
Materials of Experiment
Chitosan – has been synthesized from cocoons of a silkworm and was presented by a Research Center of Chemistry and Physics of Polymers of National University of Uzbekistan. Chitosan was following specifications: its 97% solubility in 2% acetic acid; molecular weight is 60000; the nitrogen content is 8.2%; the deacetylation factor is ≥74.4% [16].
Glutaraldehyde – has been used for crosslinking and forming hermetic and hardness granules.
Creation of Granules
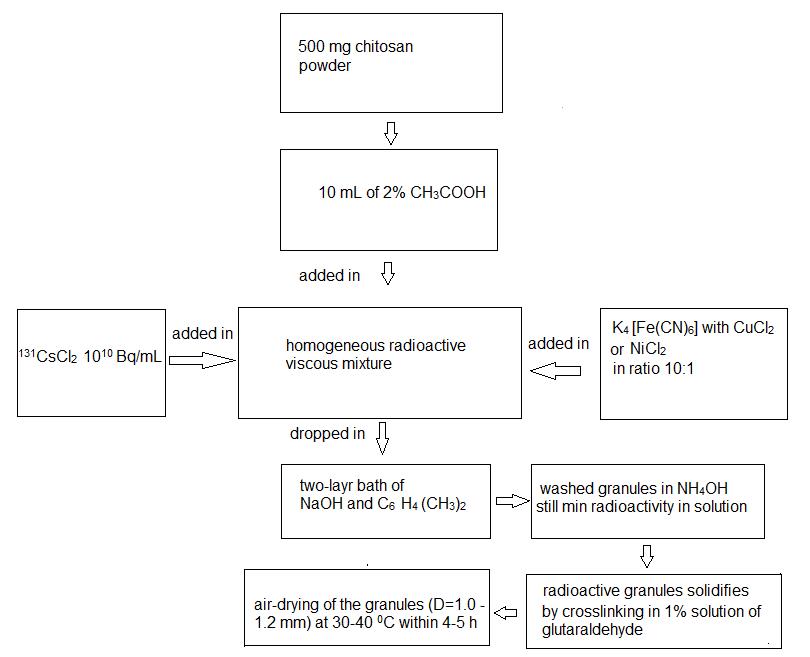
The sorption efficiency of chitosan solution to ions of 131CsCl was studied without modified additives originally. In our case we used a 2% acetic acid by dissolving chitosan powder weighing 500mg in 10ml of acetic acid with the aid of a universal stirrer. In this case the solution with necessary viscosity is formed. The results of tests as show the maximal values of sorption of the 131СsCl ions by chitosan sorbent without modified did not exceed 36%. To increase sorption efficiency, the chitosan solution has been modified by additives of potassium ferrocyanide and chlorides of transitive metals. The creation scheme of 131Cs granules at processing by modify chitosan sorbents is shown in Figure 1.
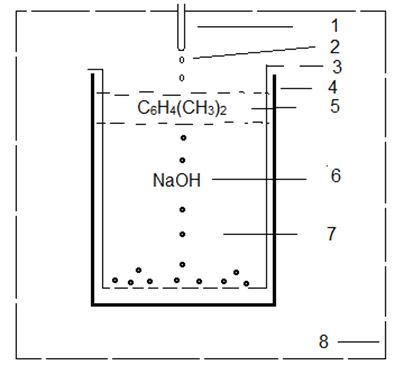
According to the given scheme, 500mg chitosan powder was dissolved in 10ml of 2% acetic acid at a room temperature in 2–3 hours. The 0.1М solution of potassium (II) ferrocyanid (K4[Fe(CN)6]) were added to 0.01М NiCl2 or CuCl2 in equal sizes and carefully stirring by using universal homogenizer stirrer in 10–15min. The chitosan solution with 131СsCl was added to potassium-nickel (II) ferrocyanid solution in ratio 10:1. All compounds were stirred on homogenizer device for 10–15min. The mixture (viscous, homogeneous and radioactive) has been fitted in the syringe. The granulation processes were carried out by passing the radioactive drops through an air at first, then through of two-layer solution in a setting bath. The diameters of formed granules depend on the internal diameter of needles (0.5±0.05 or 1.0±0.1 mm). The schematic view of setting bath is shown in Figure 2.
The radioactive drops (2) from needle (1) are dropped to internal bath (3) that fixed in setting bath (4). The baths a filled with two-layer solution, the upper-layer (5) is xylene [C6H4(CH3)2] and under-layer (6) is alkali (NaOH). Bottom of the internal bath (3) are having a holes with diameter <0.5mm and (3) may be take out from (4) for separated only 131Cs granules (7). All steps of granulating are protect with lead glass (8) for radiation safety of personal. The availability of a surface tension of the alkaline solution leads to changing of granules from ball-shape of in the granulating process. To decrees of a surface tension of upper layer, were carried out the tests by using following organics: hexane, xylene and isopropyl alcohol. It is experimentally established that in granulating process isopropyl alcohol and hexane have not desirable effect, as comparing with xylene.
The influence of various concentration of the alkaline solutions: 1М NaOH, 3M NaOH and 1–3M NH3 for formation a spherical shape were investigated also. As a result it was ascertained the shape of the granules that has aspheric form by using of 1–3М NH3. At using the 3M NaOH to led very increasing washing process up to the neutral solution. Therefore, the solution of 1М NaOH was chosen as a working alkaline. It is experimental established an optimal condition for formation spherical shapes of the granules, which are following: radioactive drops should be (Figure 2) passed through 2.5–3cm air distance, and through 1.0cm upper-layer of xylene [5], then through 20cm layer of 1М NаОH [6].
The obtained granules were washed in 0.1M solution of ammonia. Then granules were to put to crosslinking in solution of glutaraldehyde to getting necessary hardness and hermeticity of granules. By tests was determined an optimal sewing concentration and sewing time, that were 1.0% solution of glutaraldehyde and 5–10min, accordingly. After end of crosslink, the granules were eliminating from solution and carry out drying by using hot air. The photo view of X-emitter granules about of 1.0mm diameters have been presented in Figure 3.
As a see from Figure 3, shapes of biopolymer granules of 131Cs are being make ball sphere with about 1.0±0.1 mm diameters and initial radioactivity of granules were (14.8±0.74)·107 Bq. The biopolymer granules of 131Cs were sufficient durability and hermeticity.
Results and Discussion
The sorption values of 131CsCl ions in chitosan sorbent modified by potassium ferrocyanide and chloride metals were determined by measuring of the residual radioactivity of solutions. Results of measurements are presented in Table 1. As shown in Table 1, the maximal sorption 100% for carrier-free 131Cs is reached at modified chitosan solution with (K4[Fe(CN)6]) and NiCl2 or CuCl2. Therefore, the decreasing of sorption degree was observed in all cases, when we add a cold cesium into solution.
It should be noted the X-emitter granules, modified by cyanides are intended for use in brachytherapy. An average fatal dose of CN– is a ≥1.52 mg/kg for human body [17]. In our case the content of CN– made up 0.15 mg/granule or 0.11 mg/kg to body of patient. As you see the dose CN– does not exceed admissible values [17].
The magnitude values of sorption degree of the Cs2+ ions by chitosan sorbents were determined by the following relationship:
Where S is sorption degree, %; Asol.ph and Ainit. are radioactivity of the solid phase and initial activity of the water phase, imp/s.
The distribution coefficient D(ml/g) was calculated by the following equation [5,14]:
Where Asol.ph and Awat.ph are radioactivity of the solid phase and water phase of the sorbents in imp/s; Vwat.ph is volume of the water phase, ml; msor is sorbent mass in g.
The washing process of formed granules in distilled water, desorption of 131Cs and radionuclide impurities was do not observed. The X-ray activity of the granules were determined by measuring on Si(Li) detector. The each radioactivity of the 5 granules were 9.84; 9.73; 10.24; 10.06 and 9.92·106 Bq with deviation ±4–6% after 40 days.
The angular distribution of dose activity of the granules was measured in the air, water and the biological tissue by using universal dosimeter FH-40LG Eberline. The dose rate (cGy/h) of the granule in different media and distances are presented in Table 2. As shown from Table 2, the dose values of the granules in air, water and tissue are very small differences.
As known the normal volume of the prostate glands are being 40-50cm3. However in practice the volume size of prostate can be has more because of disease. In table 3, are given calculated data of dose rate per granule, and number of implanted granules and total doses according to the type and the size of prostate for cancer treating. The need data was taken from [10,18].
It is seen from Table 3, the average number of the X-ray granules of biopolymer that needed for treat prostate cancer makes up 70-100 pieces of granules with total activity of 1.3-1.9Gy/h.
Conclusions
On basis of the 131CsCl ions and chitosan sorbent with modify additives were created the X-ray biopolymer granules that having biocompatible and biodegradable properties. For creation X-ray granules were elaborated a double-layer bath technique with xylene and alkali solution. An optimal mixture of the modify additives in chitosan sorbent for selective and full precipitation of the 131CsCl ions have been determined. Was find optimal condition to obtain the hard and hermetic granules in solution of glutaraldehyde. In future planned carry out a preclinical trial on implanting these granules.
Acknowledgment
The authors thank professor S.SH. Rashidova and colleagues at the Chemical-Physical Polymer Research Center for supplying chitosan samples. We thank also professor D.A. Zareddinov and Dr. A. Fazilova for carrying of ultrasonic imaging of the granules in organic tissues from Tashkent Institute of Advanced Medical Studies.
- Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym. 46: 1-27.
- Edith IA, Ikhuoria MA, Augustin OO (2006) Effect of solvent type and drying method on protein retention in chitosan-alginate microcapsules. Tropical J Pharm Research, 5: 583-8.
- LianYan W, Yong-Hong G, Qing-Zhu Z, Guang-Hui M, Yin-Hia W, Zhi-Guo S (2006) Prepetition and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids and Surfaces B: Biointerfaces, 50: 126-35.
- Metwally E, Elkholy SS, Salem HAM, Elsabee MZ (2009) Sorption behavior of 60Co and 152+154Eu radionuclides onto chitosan derivatives. Carbohydrate Polymers, 76: 622-31.
- Egorin A, Tokar E, Zemskova L (2016) Chitosan-ferrocyanide sorbent for Cs-137 removal from mineralized alkaline media. Radiochica Acta. 104: 657-61.
- Cai H, Pang F, Zhang W, Peng F, Li L (2016) Preparation of 131I-collagen-chitosan microspheres for interventional radionuclide therapy. J Nucl Med. 57: 1461.
- Park KB, Kim YM, Shin BC, Kim JR. Therapeutic application of new holmium-166 chitosan complex in malignant and benign diseases, 569-80.
- Shirvani AS, Mahmoodabadi A, Bahrami SA, Jalilian A, Mazidi M, Afarideh H (2011) Preparation quality control and biostribution studies of 165Dy-chitosan for radiosyno-vectomy. Nucleonika, 56: 277-82.
- Zolghadri S, Mirzaei A, Athari-Allaf M, Yousefnia H, Jalilian A (2015) Devolopment of 90Y-chitosan as a new agent for radiosynovectomy. J Radioanal Nucl Chem. 306: 47-55.
- Airmpilia CI, Dale RG, Coles IP, Jones B, Antipas V (2003) The determination of radiobiologically optimized half-lives for radionuclides used in permanent brachytherapy implants. Int J Radiation Oncology Boil Phys. 55: 378-85.
- Roeslar H, et al. (1990) Superselective 90Y-resin embolization therapy of solid tumors. Eur J Nucl Med. 16: 439-42.
- Andrews JC, Walker SC, et al. (1994) Hepatic radioembolization with 90Y containing glass microspheres: Preliminary results and clinical follow-up. J Nucl Med. 35: 1637-44.
- Mumper RJ, Ryo UY, Jay M (1991) Neutron-activated 166Ho-poly(L-lastic acid) microspheres: Potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 32: 2139-43.
- Khujaev S, Vasidov A, Markelova EA (2013) Extraction of the 131Cs from neutron irradiated barium oxide under microwave radiation. J Radioanal Nucl Chem. 298: 435-8.
- Vasidov A, Khujaev S, Markelova EA (2010) Determination of the radionuclide impurities in 131Cs solutions. Atomic energy, 109: 330-2.
- Rashidova SSh, Ryziev FI, Vokhidova NR, Klicheva OB (2013) Creation of the non-waste technology for chitin-containing raw. The bull invent of Uzbekistan, 8: 21.
- Toxicological profile for cyanide. www.atsdr.cdc.gov
- Mark K Murphy, R Kim Piper, Lawrence R Greenwood et al. (2004) Evaluation of the new cesium-131 seed for the use in low-energy x-ray brachytherapy. Medical Physics. s31: 1529-38.


Tables at a glance
Figures at a glance