Anatomy of a Discovery – Cuprous Bromide/Methyl Formate Catalysed Methoxylation of Bromoaromatics
Received Date: March 08, 2025 Accepted Date: April 08, 2025 Published Date: April 11, 2025
doi:10.17303/jocs.2025.3.102
Citation: Rob Bryant (2025) Anatomy of a Discovery – Cuprous Bromide/Methyl Formate Catalysed Methoxylation of Bromoaromatics. J Org Che3m Chem Sci 3: 1-11
Abstract
Chemistry is an inexact science, so that when exploring new synthetic pathways, the experiments that do not proceed according to plan can often be just as instructive as the successes. This paper explores issues relating to the discovery of new chemistry and the varying effectiveness of the dissemination of this information to the scientific community.
Discovery: Careful observation of the negative aspects of the well-known, but inefficient Ullmann reaction being applied to an industrial process led to the development of a greatly improved process. The power of this novel catalytic synthesis was then demonstrated across a wide range of bromoaromatic compounds. These reacted with methanolic sodium methoxide and a novel cuprous bromide/methyl formate catalyst (formed in situ) giving high yields of good quality methoxyaromatics.
Significance of this novel chemistry: The original target was a pivotal intermediate to manufacture trimethoprim, which was an important antibacterial at the time. Mono- and polymethoxylated aromatic compounds (and their derived phenols) feature commonly in natural products, many of which have been used as starting points for pharmaceutical active ingredients (APIs). A useful review of methoxy group APIs has recently been published [22].
Patents and their limitations in spreading important chemical synthetic discoveries: The sad fact is that patent protection is designed to bury the results of research, rather than advertise and spread the news of important new technologies. This paper reviews how the novel methoxylation catalyst has not been taken up as it ought to have been and comments on the more recent techniques offer less efficient and much more costly solutions to a problem that was solved forty-five years ago.
keywords: Chemistry; Ullmann Reaction; Catalytic Synthesis; Bromoaromatic Compounds; Methoxylation Catalyst
Introduction
Not all publications in the chemical literature are equal. A process patent is generally designed as an obfuscation, intended to avoid casual scrutiny, rather than announce the exclusivity to the world. A further way of avoiding much interest is to design an unreadable abstract. As an example, the British Patent GB2089672 (A):
The present invention provides a process for the preparation of compounds of the general formula wherein A represents the residue of an aromatic ring forming the whole of a part of a monocyclic or polycyclic, at least partially-aromatic, carbocyclic and /or heterocyclic ring system, optionally bearing one or more other substituents inert to the reactants here employed, and R represents an optionally-substituted alkyl, alkenyl, alkynyl or benzyl group having up to 12 carbon atoms. According to the process of the invention such compounds are prepared by causing an aromatic compound of the general formula: wherein A is as defined above and X represents a chlorine, bromine or iodone atom, to react with an alcoholate of the general formula: in which M represents an alkali metal or an alkaline earth metal atom; n is the valency of M; and P is as defined above, in the presence of a catalytically- effective amount of an active catalyst mixture comprising: (i) a formic acid ester of an organic alcohol having the general formula: R2-O-CO-H (V> in which R<2> represents an optionally-substituted alkyl, alkenyl, alkynyl or benzyl group having upto 12 carbon atoms; and (ii) a cuprous salt; in a liquid medium which is a solvent for the catalyst mixture and in which the compound of formula II is at least partially soluble, under substantially anhydrous conditions and a non-oxidising atmosphere, to yield the desired corresponding compound of formula VI.
In order to convey something of the impact of this innovative chemistry, which generated USD millions of sales for the author’s employer, a more fitting abstract might have been:
A broadly applicable technique for replacing a bromide by a methoxyl substituent at an aromatic ring. This chemistry works on an industrial scale as has already been used to produce several important pharmaceutical ingredients. The cheap cuprous bromide catalyst is used in methanol at reflux temperatures and reaction times are rarely much greater than four hours. Yields are generally over 80% and the purity of the readily isolated products greater than 98%.
This paper describes this aryl bromide methoxylation reaction in some detail and reviews how its significance to such synthetic transformations has never been fully realised.
Discussion
One of the more frustrating aspects of being employed in industrial chemistry is the need to maintain secrecy about the results of the work being undertaken. The work I carried out between August-October 1980, which was later been patented [1], appears to have been largely overlooked by later authors. Its use on a plant scale by the company for which I was working generated USD millions of sales. My intention in publishing a description of this chemistry is to try to alert organic chemists to the existence of a very useful reaction, which really should be better known.
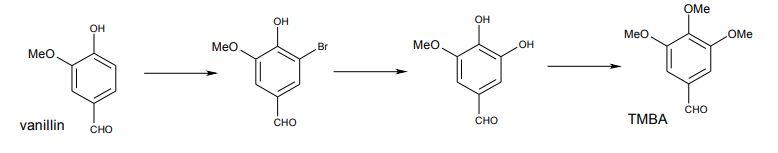
My work at the North-Eastern England-based fine chemical company, Sterling Organics, involved offering solutions to synthetic challenges that involved coming up with something a little more original. The company had been manufacturing an intermediate for the Glaxo anti-infective, trimethoprim, for many years. This pivotal aromatic intermediate was 3,4,5-trimethoxybenzaldehyde (TMBA). Produced in a three-stage process starting from vanillin, the second stage was a relatively inefficient, impurity-forming metallic copper-catalysed hydroxylation carried out in aqueous caustic soda at around 140 deg C. The original development of the process in use was not known, but it followed chemistries described in recent literature papers [2,3]. The plant process (outlined below) had been recently investigated by a team of four plant chemists over a period of 5 months.
They established that only minor improvements could be made by changing the reaction parameters. The crucial problem was that the reaction was highly heterogeneous, with both copper and bromovanillin being insoluble in the aqueous solution. The main side-reactions were:
- Debromination, giving up to 15% reduction to vanillin. This was independent of the concentration of caustic soda.
- Tarry by-products, which could only be reduced by decreasing the concentration of the aqueous caustic soda, which reduced the volume efficiency.
- Slight yield increases were achieved by lowering the reaction temperature, but this also extended the reaction time (already a longer than ideal 30 hours).
Addition of 5-10% by volume of organic bases (quinoline, pyridine, morpholine, piperidine and triethylamine were tried) was found to improve the reduction ratio from 5:1 to 7-8:1. This was not a cost-efficient solution, so it was abandoned. Before starting practical work, a thorough search of the literature was undertaken [4-7]. It became clear that, at that time, little was known about copper-catalysed substitution at aromatic halogen substituents. Generally, the conditions described would be best described as “witches’ brews”, containing metallic copper or its salts heated in basic solutions at relatively high temperatures. The exact state of subdivision of the copper powder was important and variable yields were the norm (another unhelpful fact-of-life on the plant-scale process). It became clear that it was well-established that:
- The competing reactions (reduction versus substitution of the halogen – see scheme) were thermodynamically close.
- Aryl chlorides followed the reduction pathway to a far greater degree than aryl bromides.
- In polysubstituted methoxyaromatics, reduction was favoured (not helpful in the case of bromovanillin).
- The more hindered alkoxides gave more reduction (tBuO- > iPrO- > CH3O-).
- Highest yields of the desired substitution were favoured in high-boiling aprotic bases, such as dimethylformide5 and 2,4,6-lutidine. Expensive options for the current case. Cuprous salts were better than cupric or metallic copper.
The plan for developing a more successful process was based upon three basic conclusions:
- The very insoluble bromovanillin must be brought into far more intimate contact with the other reactants.
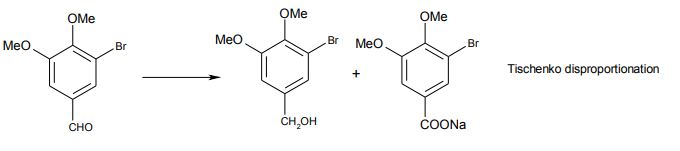
- Methoxylation, rather than hydroxylation, was preferred. This would also reduce the quantity of dimethyl sulphate needed in the final stage (not so far mentioned, but this stage also suffered from competing side-reactions, caused by the problem that the reaction conditions led to substantial hydrolysis of the dimethyl sulphate). Note also that methylation of the phenol prior to methylation would also fail, since the Tischenko disproportionation would predominate without the phenol being present)
Reported methoxylation conditions included [5]:
- Sodium methoxide in dimethylformamide (DMF), with cuprous iodide
- Methanolic caustic soda in DMF, in the presence of calcium oxide and cupric chloride
- Sodium methoxide in DMF, with cuprous chloride
- Sodium phenoxide, with a cuprous halide-triphenyl phosphine complex.
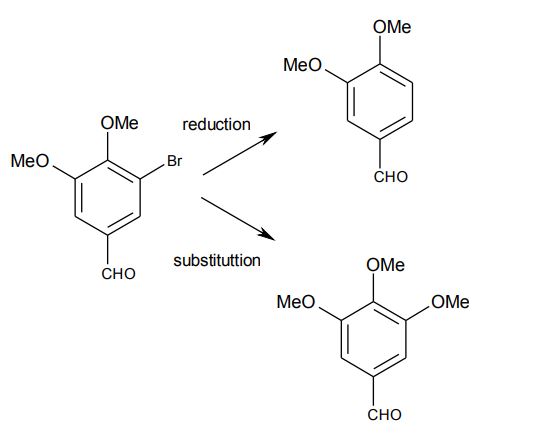
There was not much help for our current purposes, since DMF would be far too costly. So, the initial reactions were carried out using methanolic sodium methoxide and cuprous bromide. Heating a suspension of bromovanillin in this mixture at reflux, produced no useful results. So how could it be brought into greater contact with the solution of methanolic sodium methoxide?
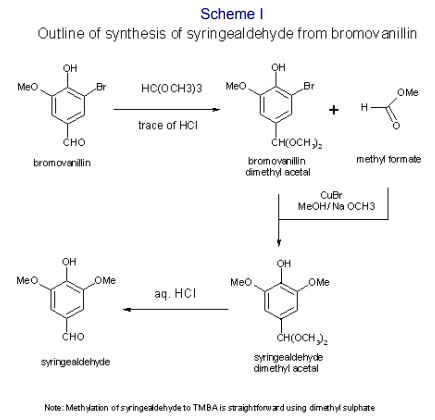
An idea was tried. It turned out that the dimethyl acetal of bromovanillin, formed readily with trimethylorthoformate (TMOF), using a trace of concentrated hydrochloric acid. The exothermic reaction quickly dissolved the suspension within two minutes. Leaving a flask containing the warm solution overnight rewarded us with huge transparent crystals (2-3 cm long). What chemist could resist isolating the crystals from the mixture?
After characterising the crystals and confirming their identity to be the dimethyl acetal of bromovanillin, it was reacted with the usual methoxylation mixture solution. Even after heating under reflux for 24 hours, a tlc showed that no reaction had occurred. This disappointing result might have led to the project being shelved. However, coming back to the problem with a fresh mind (after a summer break) and a freer hand, I decided to carry out the reaction by forming the acetal in situ with trimethyl orthoformate and then introducing it, via a dropping funnel, to the methanolic sodium methoxide and cuprous bromide mixture at reflux. This meant that there was no exposure to air and the by-product methyl formate remained in the mixture. In retrospect, this was crucial to the exciting result of this experiment. Within two hours at reflux, all the cuprous salt dissolved, yielding a straw-coloured solution, which upon cooling became blue, as air was drawn in under cooling. Work-up under aqueous acidic conditions (which also hydrolysed the acetal) allowed a crystalline product to be isolated, which was shown to be syringaldehyde (4-hydroxy-3,5-dimethoxybenzaldehyde). The yield was around 95%. Further experiments established the following facts and vital aspects required for a successful reaction:
A process outline is provided below. The chemical stages are shown in Scheme 1.
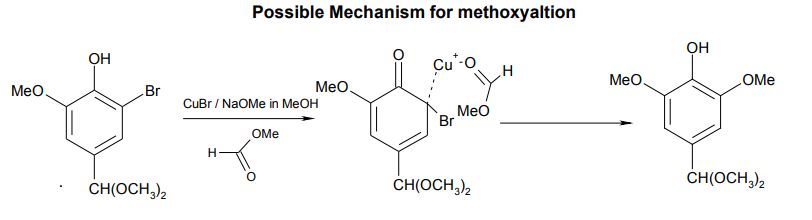
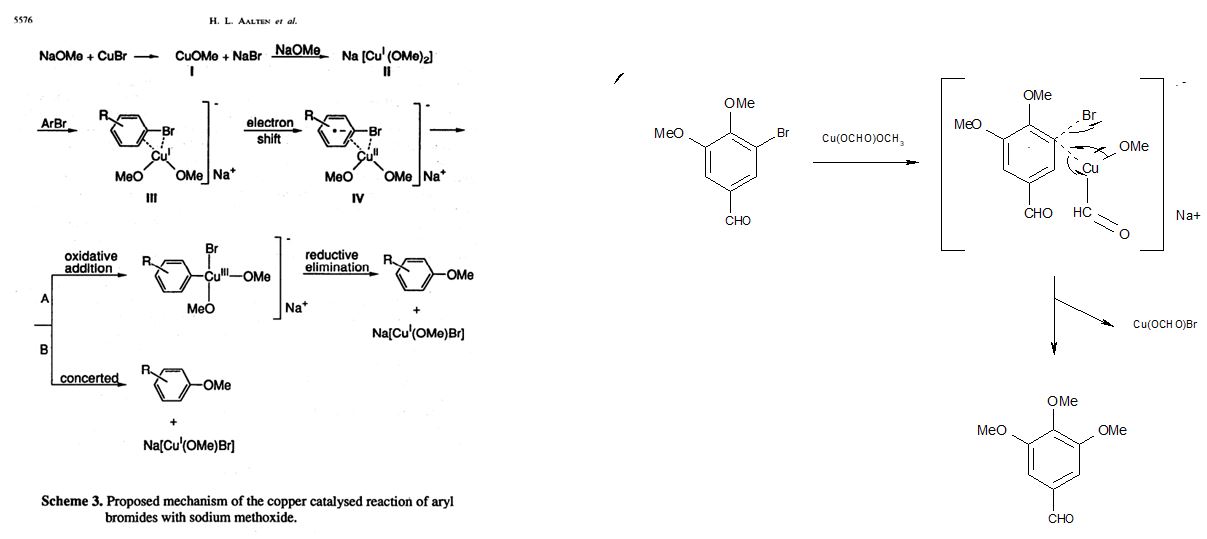
- Even traces of water, sodium hydroxide or air all slowed the reaction. The catalyst that was forming in situ, appears to be a complex of cuprous ion with methyl formate, which was readily hydrolysed by water or hydroxide and/or oxidised by air (see proposed structure in the diagram below).
- Carbon monoxide was evolved during the reaction (one equivalent to the formate present).
- Commercial sodium methoxide was entirely useless, since it contained substantial amounts of hydroxide, so the methanolic sodium methoxide had to be prepared by addition of sodium metal to methanol (fresh industrial methanol from a 200 litre drum is suitable, but laboratory supplies may contain water, since methanol is deliquescent).
1. In a secondary reaction vessel, dry bromovanillin (1.0 moles) is suspended in methanol and stirred vigorously. Trimethyl orthoformate (1.3 moles) is run into the mixture and then a trace of concentrated hydrochloric acid (1-2 ml) added.
2. The mixture heats up to ca. 50 deg C under a reflux condenser and purged with dry nitrogen (optional, if the mixture is warmed without stopping). It is maintained at this temperature to avoid crystallisation of the diacetal. This solution is ready for transfer to the main reaction vessel.
3. In the main reaction vessel, sodium metal (2.5 – 4.0 moles) is dissolved in methanol containing < 0.1% water. The solution of sodium methoxide is cooled to 30 deg C and 20% of the diacetal solution run in from the secondary reaction vessel.
4. Cuprous bromide (0.05 – 0.2 moles) is added and the reaction slowly brought to reflux, with efficient stirring needed to avoid foaming (some carbon monoxide is emitted at his point). The balance of the diacetal mixture is run in gradually over 2 hours and then the mixture maintained at reflux for a further 10 hours. The straw-coloured mixture, which is now generally almost homogeneous, is cooled to room temperature. As air enters, the mixture becomes blue.
5. The condenser is set to distillation and the methanol removed under reduced pressure (on a small scale a rotary evaporator is ideal).
6. Dilute (2N) hydrochloric acid is added to the solid residue and then the aqueous suspension is extracted twice with isopropyl acetate (preferred) or toluene. The organic layers are combined and the solvent removed.
7. The residue is recrystallised in a minimum of methanol, cooled and a pale-yellow product is filtered off. A yield of > 90% of syringaldehyde of >99% purity is obtained.
Conversion of syringaldehyde to TMBA by dimethyl sulphate turned out to be straightforward. This original process was eventually developed on a large scale and the three stages gave the following isolated yields on 50-80 kg batches:
vanillin -> bromovanillin 98% / bromovanillin -> syringaldehyde 91% / syringaldehyde -> TMBA 95%
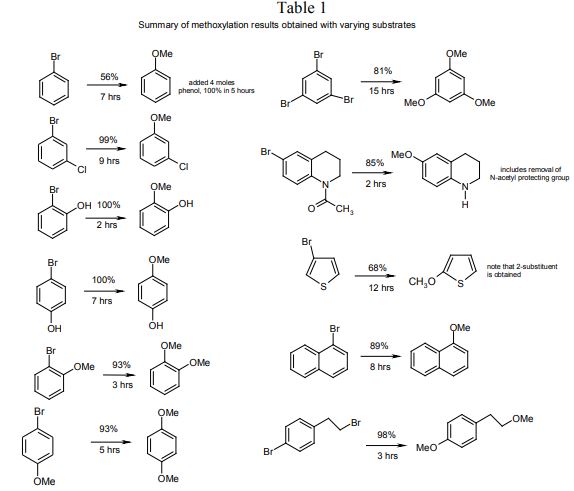
The process was agreed to be worthy of further investigation and was found to be applicable to a wide range of aromatic bromides. Generally, the yields were good to excellent. Other cuprous salts give similar results, but cupric salts were shown to be inactive. Chloroaromatics failed to react at all. In Table 1, a representative summary of compounds that were tested is shown.
Two more of these were of immediate commercial use: 1,3,5-trimethoxybenzene (TMB), an intermediate already being used to produce buflomedil and 6-methoxytetrahydroquinoline (6- MTHQ), an intermediate under development for making rosoxacin.
The cost of the existing synthesis of 6-MTHQ was rather high and by using the new process, the final active ingredient could be made at the same cost as the original method for making 6-MTHQ, a spectacular improvement.
Although the TMB process was one of the slowest methoxylations, it was nevertheless commercially viable. The older route for making TMB started from 2,4,6-trinitrotoluene (TNT), an intermediate being sourced at the time from only one supplier, which was closing production of this famous explosive for reasons of safety.
The important facts about the catalytic process that emerged from these reactions were:
- As the electron density on the aromatic ring increases, the rate of methoxylation increases. This is the opposite to the rule that applies to normal nucleophilic substitutions, showing that the mechanism must be different.
- The activating influence of aromatic substituents is greatest for those ortho to the bromine group and least to those meta (o- > p- > m-).
- After the publication of the patent, I received several comments from chemists stating that the reaction “didn’t work”. In all cases, it turned out that commercial sodium methoxide had been used, or poor control of the oxygen levels had occurred. Unfortunately, many chemists thought they knew better than the author how to conduct the reaction. This even happened in Sterling Organics’ own production plant!
Post publication comment: Brandsma et al in 1990 (Syn Comm. Page 213) reported the reaction between 3- bromothiophene and methanolic sodium methoxide, in the presence of cuprous bromide at 100 deg C, gave an 88% yield of the expected 3-methoxythiophene. It may be that my original NMR interpretation (details were given in the Sterwin patent1) was flawed or that the different conditions gave different results.
Post-patent literature search (selected findings, January 2025)
It is now 53 years since my patent [1] was published and it seemed a good idea to find out what progress in the area of cuprous salt catalysed methoxylations had been made in the interim. Sadly, little useful progress occurred. The one exception is the paper published by Capdeveille and Maury in 1993, which specifically cites my 1980 patent as being of significance [7a]. Of particular note is the almost total lack of patent citations in the papers on aryl bromide methoxylations. It would appear that commercially useful chemistry does not qualify as academically worthy enough to be taken in consideration. The following relevant papers, published since 1982, have been identified as worthy of note:
Strauss et al [8]
Review of earlier papers covered only the 21st Century. Majority of cited references employed complex chelating agents and/or problematic solvents such as HMPA and DMSO. Strauss' own work covered a wide array of multifunctional starting bromides, but used 10-20% moles of a complex diaryldiamine ligand, with DMSO favoured as the solvent. Though effective, it would seem that the reactions would be quite costly to operate.
James Lindley, Copper assisted nucleophilic substitution of aryl halogen [9]. On the face of it, this review should be a useful source of information, being published just two years after the RB patents, which are not referenced, however. The short section on displacements by oxygen nucleophiles also contains inaccurate generalisations and the review of the mechanisms is unhelpful.
Aalten et al (including Andeno BV's Roger Sheldon), Copper catalysed reaction of sodium methoxide with aryl bromides [10].
This review appeared to be much the most relevant of the ones identified, and so it proved. Again, no mention of the RB patent is made. The proposed mechanism, largely based upon a series of reactions between sodium methoxide and bromobenzene, is similar to the musings our team had. If their proposal has any merit, then the posited cuprous dimethoxide catalyst would be replaced by cuprous methoxide-formate in the case of the RB catalyst (below right). This would greatly accelerate reactions by stabilising the intermediate radical anion, IV, shown in the Tetrahedron figure (reproduced below, left). Interesting paper, although it fails to identify the best conditions for bromide displacement.
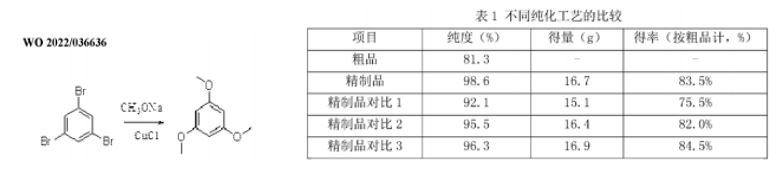
Preparation of 1,3,5-trimethoxybenzene, Liu Jianming, Jiangsu Kanglong Pharmaceutical Company, China [11].
This patent covers one of the important commercial processes claimed in the1982 Sterwin patent, the protection for which had expired in the 1990's. Since the text is unhelpfully written in mandarin, only a sketchy understanding of the claims was possible. However, yields of 75- 85% were claimed for the methoxylation of 1,3,5-TMB (table of results shown below). Unlike the academic papers, the Sterwin patent is listed (together with the Andeno DMF patent, of which the process described is a close copy) as relevant! All other references are to Chinese authors' work; some sort of recognition.
A ligand-free method for methoxylation of unactivated bromides by use of CuCl/HCOOMe/MeONa/MeOH system, Liu Hong-Wei, Research on Chemical Intermediates [12].
Although this online-published paper appears highly relevant, only the references and a brief abstract are available without spending GBP 29.00. Since it appears to be a rip-off of my work, I was not prepared to spend the cash.
Many other literature references were found, but none are as relevant as the ones detailed above. Unfortunately, a copy of the highly relevant Capdeveille/Maury paper [7a] could not be obtained, with just an abstract being seen. This paper and the patents quoted the RB catalyst patent as a relevant precedent. A list of these is presented in the bibliography.
Acknowledgements
The work described above was undertaken in Autumn 1980 in the long-term development laboratories at the Fawdon site of Sterling Organics. I must include the names of my coworkers, without whom this useful discovery might not have been made: Geoffrey Askew, William Little and Dr Peter Urben. The earlier work on the process was undertaken Dr Frank Reed and his team of plant chemists. The intellectual slack cut by the head of R&D, Dr John Harrison, was crucial for such exploratory work to be carried out. The decision to use the catalyst system for making the rosoxacin intermediate was made by Dr Maris Bite, who later became my long-term business partner. Use of what became called, the “RB catalyst” outside of the discovery laboratory was helpful to convince others to take the novel chemistry seriously. This was especially true when the technology was transferred to the plant.
Lessons Learned
This experience was an example of something that I had long believed; that useful progress in science is most often achieved as a result of following one’s hunches when the unexpected happens. The most pleasurable aspect of applying knowledge of synthetic organic chemistry to practical problems is that in one case in ten, it will produce something unexpected. Following up the unexpected can deliver returns in many cases.
- British patent GB 2089672A; R J Bryant, assigned to to Sterwin. Equivalents were granted in the USA, Ireland, Canada, Japan and Europe.
- JM Pepper, JA MacDonald (1953) Canad. J. Chem, 31: 476.
- RGR Bacon, HAO Hill (1964) J. Chem. Soc. 1097.
- Chem. Abs 70: 46554g (1968 paper written in Russian)
- Alexander McKillop, Barrie D. Howarth, Ryszard J. Kobylecki (1974) Synth. Comm, 4: 35.
- RGR Bacon, SC Rennison (1969) J. Chem. Soc. (C), 308-12
- RGR Bacon, JR Wright (1969) J. Chem. Soc. (C), 1978.
- 7a. Patrice Capdeveille and Michel Maury, Esters are effective co-catalysts in coppercatalysed methanolysis of aryl bromides, Laboratoire de Recherches Organiques de l'ESPCI, associé au CNRS, 10 rue Vauquelin, F-75231 Paris Cedex 05.
- Dr. Michael J. Strauss, Dr. Megan E. Greaves, Dr. Seoung-Tae Kim, Dr. Christiana N. Teijaro, Dr. Michael A. Schmidt, Dr. Paul M. Scola, Prof.Dr. Stephen L (2024) Buchwald Angew. Chem. Int. Ed. 63: e202400333.
- James Lindley (1984) Copper assisted nucleophilic substitution of aryl halogen, Tetrahedron report No. 163, Tetrahedron, 40: 1433-56.
- Aalten HL, Koten van G, Grove DM, Kuilman T, Piekstra OG, Hulshof LA, Sheldon RA (1989) Copper catalysed reaction of sodium methoxide with aryl bromides, Tetrahedron, 45: 5565-78.
- WO/2022/036636, Preparation of 1,3,5-trimethoxybenzene, Liu Jianming, Jiangsu Kanglong Pharmaceutical Company, China.
- Ying Guo, Si-Zhe Ji, Cheng Chen, Hong-Wei Liu, Jian-Hong Zhao, Yu-Lin Zheng, Ya-Fei Ji (2015) A ligand-freemethod for methoxylation of unactivated bromides by use of CuCl/HCOOMe/MeONa/MeOH system, Research on Chemical Intermediates, 41: 8651-64.
- Reaction of 5-bromovanillin with sodium methoxide, McIvor and Pepper, Can. J. Chem. 1952, 11: 298-302
- Solvolysis of 5-bromovanillin to syringaldehyde in the presence of Cu catalyst, Borgaonor et al, J. Chem. Tech and Biotech. 1984, 34B: 446-52
- Regiospecific alkoxylation of phenolic aldehydes, Puri et al, Ind. J. Chem. 1985, 24B: 294-95
- Iodoaromatics, Larkins, Eastman Kodak, US. 1990, 4: 918-241.
- Diaryl ether condensations, Marcoux et al, MIT, US. 2002, 6: 395-939.
- Expeditious synthesis of syringaldehyde from p-cresol, Tripathi et al (2010) Ind. J. Chem, 49B: 379-81.
- Noribogaine, Robert Moriarty, DemeRX, US. (2014) 8: 877-921.
- Pharmaceutical-oriented methoxylation of aryl C (sp2)-H bonds using Cu catalysts, Zhang et al, SynLett, published in 2018 online.
- Chinese patent CN 113,666,826 (2021). no english language abstract, but structures show this patent to be relevant.
- The role of the methoxy group in approved drugs, Debra Chiodi and Yoshihiro Ishihara, Eur. J Med Chem, 2024, 273: 116364.

Figures at a glance