Synthesis and Spectroscopic Characterization of Novel Imidazole-Based Semicarbazone Schiff Base Ligands
Received Date: July 01, 2025 Accepted Date: July 12, 2025 Published Date: July 15, 2025
doi: 10.17303/jocs.2025.3.104
Citation: Mohammed Bahreldin Hussein, Eiman AA Elshaygi (2025) Synthesis and Spectroscopic Characterization of Novel Imidazole-Based Semicarbazone Schiff Base Ligands. J Org Chem Chem Sci 3: 1-13
Abstract
Semicarbazone derivatives are important in organic synthesis and they are used in evaluating new product that possesses different biological activities. Such as antibacterial, antifungal, antitumoral, antiviral, anticancer anticonvulsant, antidepressant and other biological activity. In this study, four new: 4-methyl-5-imidazolecarboxaldehydesemicarbazone (C1), 4-imidazolecarboxaldehydesemicabazone (C2), 1-methyl-2-imidazolecarboxaldehydesemicarbazone (C3) and 1-methyl-5-imidazolecarboxaldehydesemicarbazone (C4) were prepared by condensation reaction of Imidazolecarboxaldehydes with semicarbazide hydrochloride. The structures of the prepared compounds were characterized on the basis of their IR, UV-Vis, ESI Mas and 1H 13C-NMR data.
Keywords: Schiff Bases; Semicarbazone; Synthesis; Spectroscopic Analysis
Introduction
Heterocyclic Schiff base compounds have been an interesting field because of their various biological poperties. A number of heterocyclic derivatives containing nitrogen and sulphur atoms provide as a exclusive and multipurpose gallows for experimental drug design [1]. Benzothiazole is one of the most important heterocyclic compounds that have received overwhelming response owing to its diversified molecular design and remarkable optical, liquid and electronic properties [2]. Benzothiazole shows various biological activities such as antimicrobial [3-5], anticancer [6-7], anthelmintic [8], anti-diabetic [9] activities. The consequential compounds, Imidazole also reveal a number of significant biological activities such as antiparasitic, fungicidal, anithelemintic, antiinflammatory, antiprotozoal and herbicidal activity [10-14]. Hence, it was thought of interest to merge both of thiazole and imidazole moieties which may enhance the drug activity of compounds to some extent or they might possess some of the above-mentioned biological activities. Semicarbazones are versatile ligands that arise the interest of researchers, not only from a coordinative point of view, but also from a pharmacological one, in recent research some imidazole derivatives such as imidazole-2-carboxaldehyde semicarbazone and thiosemicarbazone, and their transition metal complexes have been synthesized [15]. From this point of view, the objective of the present work is to prepare new derivatives of semicarbazone Schiff base of imidazolecarboxaldehydes.
Materials and Methods
All chemicals and solvents of highest analytical grade were used as received form Sigma-Aldrich and Alfa-Aesar. The FT-IR of synthesized semicarbazones Schiff bases was recorded on Vertex-183387000 FT-IR spectrometer by using KBr disk in the range 400-4000 cm-1. UV-Vis spectra in solid state was recorded on a Cary-4000. EL 05123055 UV-Vis spectrophotometer, Mass spectrum was carried out on Esquire LC-00084 electronic spray ionization (ESI) Mass spectrometer and 1H and 13C NMR spectra were recorded on Bruker AV-III 600 by using DMSO-d6 as a solvent.
Synthesis of Imidazolecarboxaldehyde Semicarbazones (C1-C4)
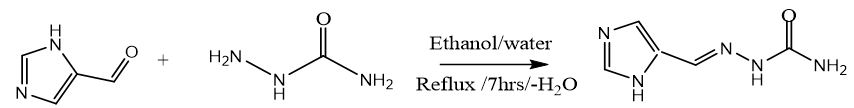
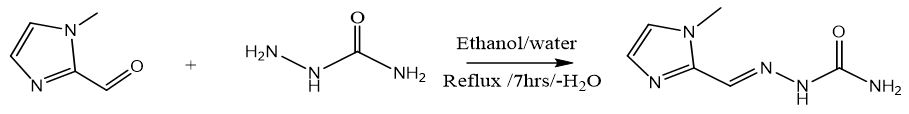
Four new imidazolecarboxaldehyde semicarbazones Schiff bases were obtained by following a general procedure previously reported by Chatterjee [16].. Since the semicarbazide was in its hydrochloride form (0.11153 g, 0.001 mol), prior to its condensation with aldehydes separately, it was neutralised by addition of NaOH in a 1:1 ratio. Thus, an aqueous solution (20 mL) of the neutralised semicarbazide was slowly added to a warm (60°C) ethanolic solution (20 mL) of imidazolecarboxaldehydes (0.001 mol). The mixture was refluxed for 7 hrs and the resulting suspension was cooled at room temperature, and then allowed for slow solvent evaporation, the crystals obtained was filtrated, washed with water and ethanol, and finally dried under vacuum.
Synthesis of 4-Methyl-5-Imidazolecarboxaldehyde Semicarbazone (C1)
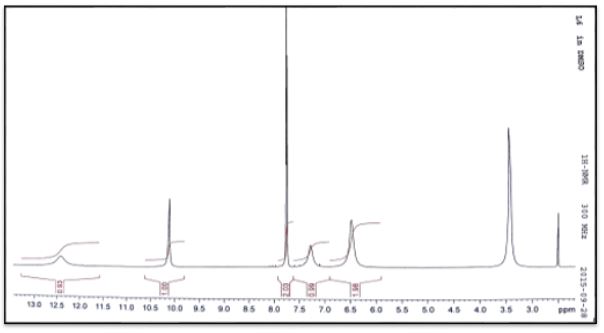
White crystals,Yield: 80%, m.p 255°C. C6H9N5O. FT-IR (KBr cm-1), 3464, 3445, ν(NH2); 3140, ν(NH); 1584, ν(C=N); 1690, ν(C=O); 1116. ν(N-N). NMR spectrum (600 MHz for 1H and 151 MHz for 13C, DMSO-d6, ppm): 1H NMR; δ=2.2 (3H, s, CH3); 7.6 (H, CH ring), 6.4(2H, s, NH2), 7.8(1H, s, =N-NH); 10.0 (1H, s, NH), 12.1 (1H, s, NH ring); 13C NMR; 135.54(HC=N); 156.93(C=O). Ms (ESI.m/z): M+ 168; Analysis for C6H9N5S (Mw 167.17). UV-Vis spectrum (λmax nm): 298.
Synthesis of 4-Imidazolecarboxaldehyde Semicarbazone (C2)
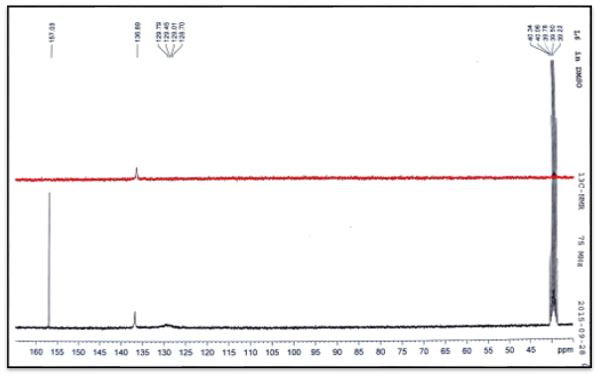
White crystals, Yield: 71%, m.p 220°C. C6H9N5O. FT-IR (KBr cm-1, 3382, 3323, ν(NH2); 3217, ν(NH); 1592, ν(HC=N); 1655, ν(C=O); 1109. ν(N-N). NMR spectrum (600 MHz for 1H and 151 MHz for 13C, DMSO-d6, ppm): 1H NMR; δ=7.3-7.8 (2H, CH ring), 6.4(2H, s, NH2), 7.8(1H, s, =N-NH); 10.2 (1H, s, NH), 12.4 (1H, s, NH ring); 13C NMR; δ=136.89 (Cq ring); 128.70-129.45(2C-H ring), 129.79(HC=N); 157.03(C=O). Ms (ESI.m/z): M+ 154.1; Analysis for C6H9N5O (Mw 153.14). UV-Vis spectrum (λmax nm): 283.
Synthesis of 1-Methyl-2-Imidazolecarboxaldehyde Semicarbazone (C3)
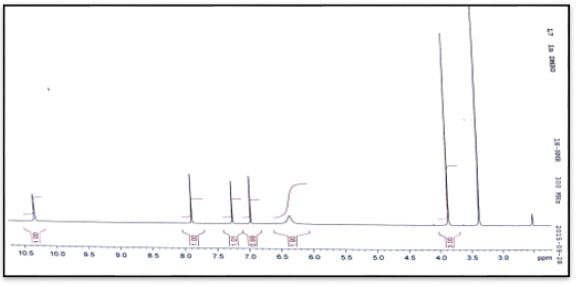
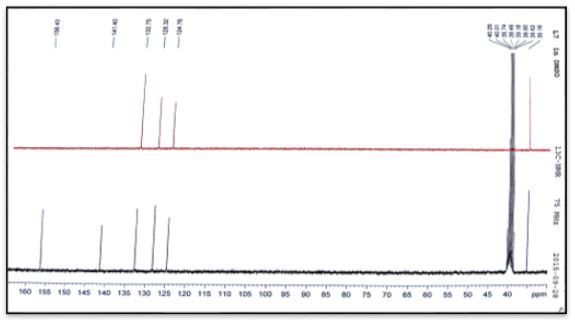
White crystals, Yield: 75%, m.p 248°C. C6H9N5O. FT-IR (KBr cm-1), 3294, 3256, ν(NH2); 3093, ν(NH); 1541, ν(HC=N); 1640, ν(C=O); 1159. ν(N-N). NMR spectrum (600 MHz for 1H and 151 MHz for 13C, DMSO-d6, ppm): 1H NMR; δ=3.8 (3H, s, CH3); 6.9-7.3 (2H, CH ring), 6.4(2H, s, NH2), 7.9(1H, s, =N-NH); 10.3 (1H, s, NH); 13C NMR; δ=35.5 (CH3);141.40 (Cq ring); 124.76-128.32(2C-H ring), 132.75(HC=N); 156.43(C=O). Ms (ESI.m/z): M+ 168.1; Analysis for C6H9N5O (Mw 167.17). UV-Vis spectrum (λmax nm): 292.
Synthesis of 1-Methyl-5-Imidazolecarboxaldehyde Semicarbazone (C4)
White crystals, Yield: 73%, m.p 240°C. C6H9N5O. FT-IR (KBr cm-1), 3471, 3309, ν(NH2); 3147, ν(NH); 1579, ν(HC=N); 1691, ν(C=O); 1120, ν(N-N). NMR spectrum (600 MHz for 1H and 151 MHz for 13C, DMSO-d6, ppm): 1H NMR δ=3.9 (3H, s, CH3); 7.3-7.7 (2H, CH ring), 6.3(2H, s, NH2), 7.9(1H, s, =N-NH); 10.2 (1H, s, NH); 13C NMR; δ=33.87 (CH3);141.24 (Cq ring); 127.36-130.78(2C-H ring), 132.26(HC=N); 156.66(C=O). Ms (ESI.m/z): M+ 168.1; Analysis for C6H9N5O (Mw 167.17). UV-Vis spectrum (λmax nm): 288.
Results and Discussion
The synthesized imidazolecarboxaldehyde semicarbazones (C1-C4) were characterized by using spectroscopic methods (IR, UV-Vis, ESI Mass and 1H, 13C NMR).
UV-Vis Spectra of Prepared Imidazolecarboxaldehyde Semicarbazones
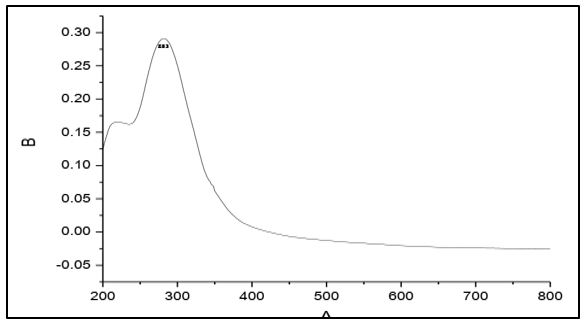
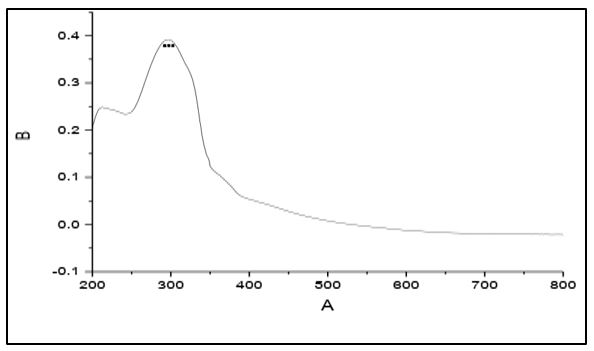
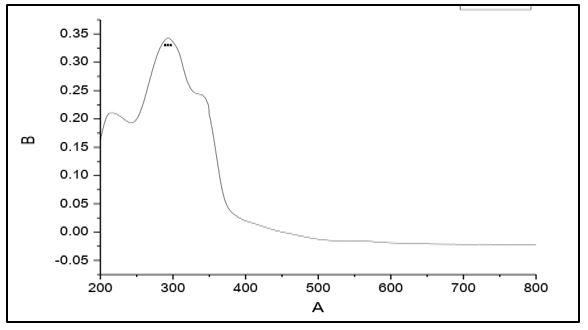
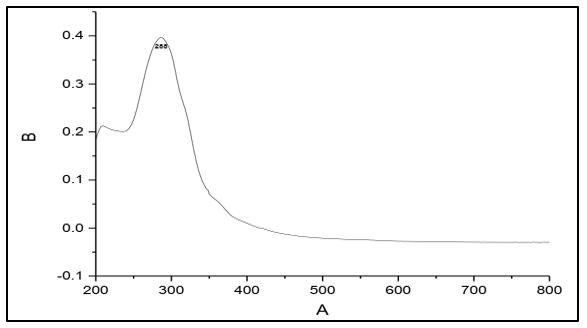
The electronic spectra of 4-methyl-5-imidazole carboxaldehyde semicarbazone (C1), 4-imidazole carboxaldehyde semicabazone (C2), 1-methyl-2-imidazolecarboxaldehydesemicarbazone (C3) and 1-methyl-5-imidazolecarboxaldehyde semicarbazone (C4). Showed that a strong absorption band at 298, 283, 292 and 288 nm respectively. These bands assigned to the π → π* transition of the azomethine group [17-18].
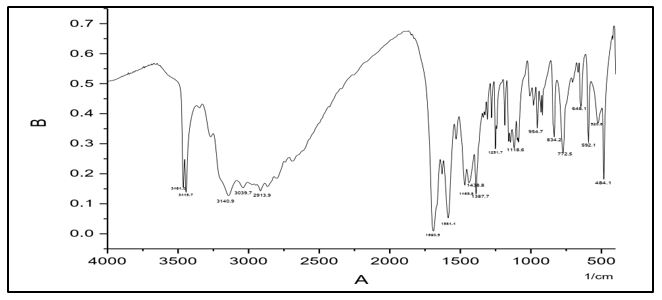
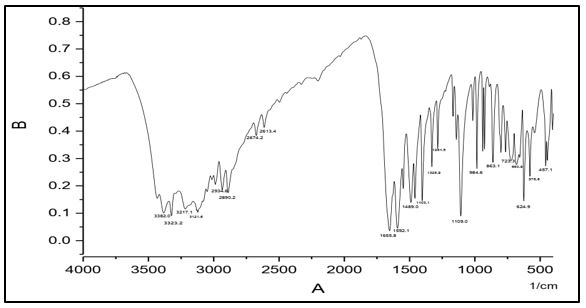
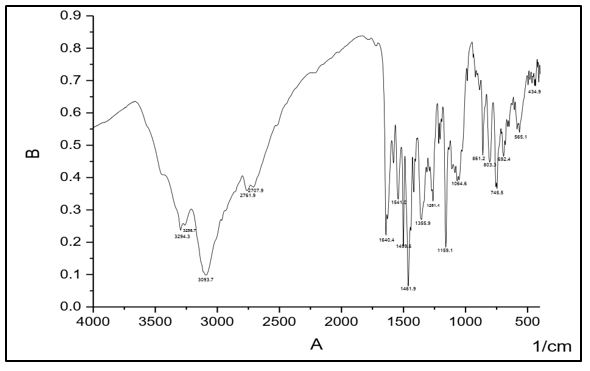
The infrared absorption bands become very useful for describing the prepared compounds. In the IR spectra, of (C1) absorption peaks at 3464, 3445 cm-1 are assigned to ν(NH2). The absorption peak at 3140 cm-1 is assigned to ν(N-H). The band at 1584 cm–1 which assigned to ν (nitrogen double bond stretching frequency, absorption band at 1690 cm-1 is attributed to ν (C=O) and peak at 1116 is assigned to ν(N-N). IR spectral data of (C2) shows absorption peaks at 3382, 3323 cm-1 whish are assigned to ν(NH2). The absorption peak at 3217 cm-1 is assigned to ν(N-H). The band at 1592 cm–1 which assigned to ν(C=N) carbon nitrogen double bond stretching frequency, absorption band at 1655 cm-1 is attributed to ν (C=O) and peak; at 1109 is assigned to ν(N-N).
IR spectral data of (C3) shows absorption peaks at 3294, 3256 cm-1 whish are assigned to ν(NH2). The absorption peak at 3093 cm-1 is assigned to ν(N-H). The band at 1541 cm–1 which assigned to ν(C=N) carbon nitrogen double bond stretching frequency, absorption band at 1640 cm-1 is attributed to ν(C=O) and peak; at1159 is assigned to ν(N-N).
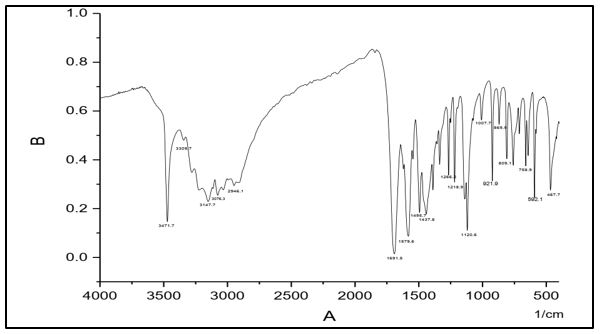
IR spectral data of (C4) Shows absorption peaks at 3471, 3309cm-1 whish is assigned to ν(NH2). The absorption peak at 3141 cm-1 is assigned to ν(N-H). The band at 1579 cm–1 which assigned to ν(C=N) carbon nitrogen double bond stretching frequency, absorption band at 1691cm-1 is attributed to ν (C=O) and peak; at 1120 is assigned to ν(N-N).
Characterization of the Prepared Imidazolecarboxaldehyde Semicarbazones by 1H And 13C-NMR Spectroscopy
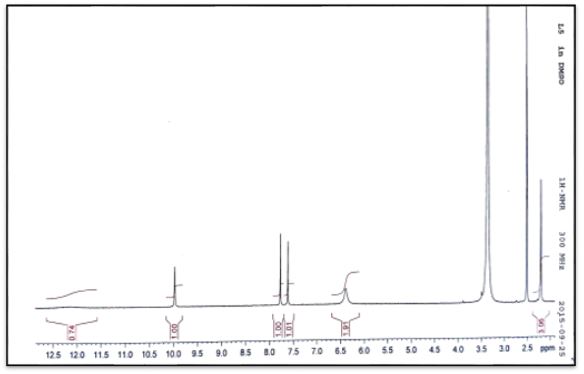
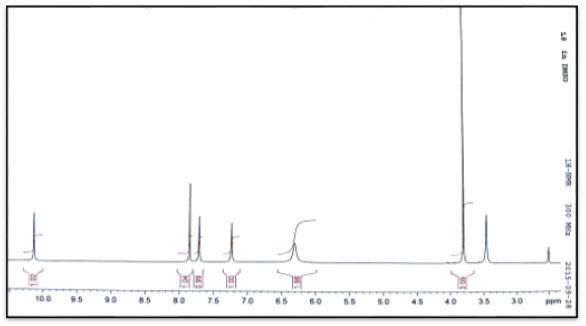
1H and 13C NMR spectra and chemical shift values of the prepared semicarebazones were record in DMSO-d6 solvent. The 1H NMR of (C1-C4) show signal at δ 7.8, 7.8, 7.9 and 7.9 ppm, respectively have been assigned to δ(HC=N) protons and signals at 6.4, 6.4, 6.4 and 6.3 ppm have been assigned to δ(NH2) protons. The signals at δ 10.0, 10.2, 10.3 and 10.2ppm assignable to δ(NH) protons. Signals at 7.6-0.0, 7.3-7.8. 6.9-7.3 and 7.3-7.7 due to hydrogen protons of aromatic ring [19].
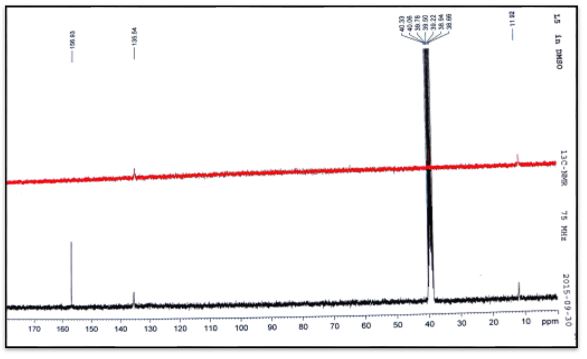
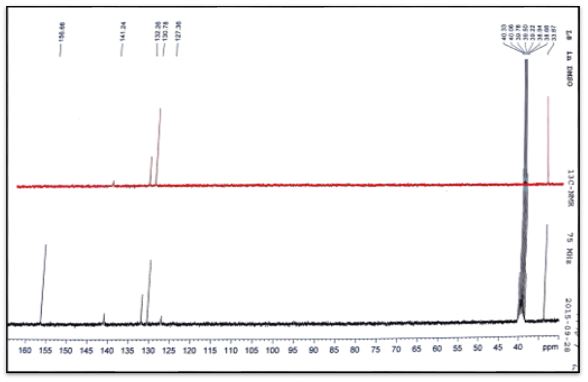
The 13C NMR analysis of (C1) the azomethine carbon (HC=N) was found at δ135.54ppm and signal at δ156.93 due to carbonyl group (C=O) [20]. The 13C NMR analysis of (C2) the azomethine carbon (HC=N) was found at δ129.79ppm. The aromatic carbon atoms in the ring appeared at δ 128.70, 129.45 and 136.89. Signal at δ157.03ppm due to carbonyl group (C=O) [19]. The 13C NMR analysis of (C3) the azomethine carbon (HC=N) was found at δ132.75ppm. The aromatic carbon atoms in the ring appeared at δ 124.76-128.32and 141.40ppm. Signal at δ156.43ppm due to carbonyl group (C=O). Methyl group carbon atom is shown at δ35.5 ppm [19]. The 13C NMR analysis of (C4) the azomethine carbon (HC=N) was found at δ132.26ppm. The aromatic carbon atoms in the ring appeared at δ 127.36-130.78 and 141.24. Signal at δ156.66ppm due to carbonyl group (C=O). Methyl group carbon atom is shown at δ35.5 ppm [19].
Conclusion
In this study, condensation reaction was adopted for preparing four new imidazolecarboxaldehyde semicarbazone Schiff bases namely; 4-methyl-5-imidazolecarboxaldehyde semicarbazone (C1), 4-imidazolecarboxaldehyde semicabazone (C2),1-methyl-2-imidazolecarboxaldehyde semicarbazone (C3) and 1-methyl-5-imidazolecarboxaldehyde semicarbazone (C4). These compounds were characterized by using spectroscopic methods (IR, UV-Vis, ESI Mas and 1H, 13C NMR,). The Prepared ligands can be use of future studies, such as metal complexation and biological activities.
Conflicts of Interest
The authors declare that there is no conflict of interests.
Acknowledgments
My great thanks to the Chairperson, Department of Inorganic Chemistry Technical University of Dresden (TUD) - Germany for providing research facilities and supervision. My thanks extended to German Academic Exchange Services DAAD for a research grant to Germany and financial support.
- Patel NB, Shaikh FM (2010) Sci Pharm. 78: 753-65
- Ha S, Koh T, Ong S, Lee T, Sivasothy Y (2009) Molbank, M, 609: 1-3
- Gupta S, Ajmera N, Gautam N, Sharma R, Gauatam D, (2009) Ind.J Chem. 48: 853-8.
- Kumbhare RM, Ingle VN (2009) Ind J Chem. 48: 996-1000
- Murthi Y, Pathak D (2008) J Pharm Res. 7: 153-5
- Kini S, Swain S, Gandhi A (2007). Ind J Pharm Sci.46-50
- Stanton HLK, Gambari R, Chung HC, Johny COT, Filly C, Albert SCC (2008). Bioorg Med Chem.16: 3626-31.
- Sreenivasa M, Jaychand E, Shivakumar B, Jayrajkumar K, Vijaykumar (2009) J Arch Pharm Sci and Res. 1: 150-7
- S Pattan, C Suresh, V Pujar, V Reddy, V Rasal, et al. (2005) Ind J Chem. 44: 2404-8
- CR Débora, ARD Angel, GS Jeferson, FS Nayane, FV Camila, CM Isolda, AT Jacqueline, B Heloisa. (2013). Molecules: 12645-62.
- FE Anderson, CJ Duca, JV (2951) Scudi, Am. Chem. Soc: 4967
- CR Marıa, CL Estefania, S Jesus, B Alessia, P Corrado, Z Franca (2000) J of Inorg Chemica Acta: 2543-52.
- T Monika, C Sulekh, (2012) Open J of Inorg Chem: 41-8
- S Kumari, SK Navin, K Seema (2012) Oriental.J. Chem: 969-74
- María C Rodríguez Argüelles, Sandra Moquea Vázquez, Jesús Sanmartín Matalobos, Ana M García Deibe, Corrado Pelizzi, et al. (2010) Polyhedron. 29: 864-70
- P Chatterjee, BV Agarwala, AK Dey, (1989) Synth. React. Inorg Met-Org. Chem. 19: 715-9
- Alsalim TA, Hadi JS, Ali ON, Abbo, HS, J Titinchi S (2013) J. Cen. Chem, 15
- Gujarathi JR, Pawar NS, Bendre RS (2013) J. Chem. Pharm. Res. 5 (7), 161-8
- Mohammed Bahreldin Hussein, Muna Mahdi Mohammed, Abdalla Gobara, Asha Fadllallah Khaleel and Awad Salim Ibrahim Holy (2021) European Journal of Chemistry. 12: 56-9
- María C Rodríguez Argüelles, Sandra Mosquera Vazquez, Jesús Sanmartín-Matalobos, Ana M García-Deibe, Corrado Pelizzi, Franca Zani (2010) Polyhedron. 29: 864-70



Tables at a glance
Figures at a glance