Empirical Estimation of the Contribution of the Frequency Shift Due to the Presence of the Van Der Waals Interaction to the Overall Shift of the Vibrational Frequencies of the O-H and N-H Groups During the Formation of O-H...A and N-H...A H-Complexes
Received Date: June 23, 2025 Accepted Date: July 15, 2025 Published Date: July 18, 2025
doi:10.17303/jocs.2025.3.105
Citation: N.L. Lavrik (2025) Empirical Estimation of the Contribution of the Frequency Shift Due to the Presence of the Van Der Waals Interaction to the Overall Shift of the Vibrational Frequencies of the O-H And N-H Groups During the Formation Of O-H...A and N-H...A H-Complexes
Abstract
On the basis of literature data, the contribution to the spectral shift of the A-H vibrational frequency of proton donor molecules due to the Van der Waals interaction WWI to the total spectral shift α of the A-H bond vibrational frequency during H-complex A-H…B formation has been determined and the dependence of the value of α on the relative enthalpy of H-complex formation has been established. To obtain these data, the available information on the shifts of A-H vibrational frequencies of proton donor molecules (alcohols and azoles) in gas, in the neutral solvent CCl4 and during the formation of H-complexes with proton acceptor molecules in the neutral solvent CCl4 was used. It was found that 1) the values of α are individual for each H-complex and can vary from 0.05 to 0.37; 2) with increasing relative enthalpy of H-bond formation, the contribution of the frequency shift due to WWI to the total shift decreases.
Keywords: Universal Interactions; H-Complexes; Azoles; Alcohols; Vibration Spectroscopy; Low-Frequency Shift.
Introduction
The concepts of H hydrogen bonding are more than 100 years old [1]. Its generally accepted molecular structure is represented as {A-H...B} (H-complex), where the fragment A-H refers to the proton donor molecule, and the atom B refers to the proton acceptor molecule. One of the reliably established descriptors of H-bond formation in the liquid phase is the presence of a low-frequency shift in the frequency of valence vibration of the A-H bond of the proton donor molecule relative to the frequency of vibration of the A-H bond in a gas or in a neutral solvent [2].
The H-complex is surrounded by solvent molecules and therefore always undergoes intermolecular Van der Waals interaction VWI with these molecules [2,3]. Thus, the observed value of the shift of the A-H bond vibration frequency during the formation of the H-complex is a consequence of the influence not only of the H-bond formation itself, but also the influence of VWI. This fact has been pointed out since the beginning of the study of the spectral properties of H-bonding, and therefore there are numerous reports in the literature on the experimental and theoretical study of the VWI effect on the vibrational frequency of the A-H bond in molecules forming H-bonds [2-26]. However, there are no works in which the contribution of the VWI shift proper to the overall shift of the vibrational frequency of the A-H bond during the H-complex formation was directly evaluated.
Taking into account the contribution of Van der Waals forces to the vibrational spectra of molecules in the condensed phase in the presence of H-bonding seems to be quite necessary and important. Obtaining information on this contribution may be of interest for a deeper understanding of the mechanism of H-bond formation, which is still widely debated between the proponents of the electrostatic [27] and covalent models [28].
The aims of the present work were: 1) to determine the contribution of the spectral shift of the proton donor molecules A-H vibrations due to the VWI α to the overall spectral shift of the A-H bond vibrational frequency during H-complex formation; 2) to study the dependence of the α value on the enthalpy of H-complex formation. To solve this questions we used the available experimental data on the A-H vibrational frequencies of proton donor molecules (alcohols and azoles) in gas, neutral solvent and during the formation of H-complexes with proton acceptor molecules in neutral solvent.
Approach for Determining the Contribution to the Frequency Shift of O-H, N-H Vibrations of Proton-Donor Molecules Due to VWI during the Formation of H-Complexes
Selection of Proton Donor, Proton Acceptor Molecules and Neutral Solvent
A number of aliphatic alcohols were considered as proton-donor molecules i containing O-H and N-H groups: methanol, 2,2,3,3-tetrafluoro propan-l-ol,
2,2,2,2-trifluoro ethanol [6] and a number of azoles: pyrrole [25, 36], indole [29, 36], carbazole [6,36], imidazole [28,36], benzimidazole [30, 36], benztriazole [27, 33, 36], and isatin [34,36]. Acetaldehyde [6], methyl acetate [6], acetaldehyde [6], methyl acetate [6] were considered as proton acceptor molecules j containing O and N atoms: diethyl ether [6], tetrahydrofuran [6], p-Dioxane [6], t-Butanole [6], methanole [6], acetonitrile [6, 36], pyridine [6], acetone [6,36], dimethylformamide DMFA [36], dimethyl sulfoxide DMSO [36]. Thus, the contribution of the shift due to the presence of the VWI to the overall shift of the vibrational frequency of the O-H and N-H groups for H-complexes of the form {O-H...O, O-H...N} and {N-H...O} was considered. Tetrahydrogen chloride CCl4 was considered as a neutral solvent. This solvent is considered to be neutral in the spectroscopy of liquid media [2,3], since the dipole moment of this molecule dCCl4 is 0. Thus, the H-complexes of all i-th proton donor molecules and j-th proton acceptor molecules in CCl4 solvent were considered in the presence of the nearest neighborhood of molecules with the same dCCl4 = 0 dipole moment. In this connection, the effect of the VWI on the frequency shift in proton-donor molecules was assumed to be the same in the absence and presence of H-complexes.
Determination of the Contribution of the Frequency Shift due to the Influence of VWI to the Total Frequency Shift of the O-H and N-H Vibrational Bands of Proton Donor Molecules During the Formation of H-Complexes
The value of α Н,i,j (1) was taken as the contribution of the frequency shift to the total frequency shift of the O-H and N-H vibrational bands of proton donor molecules during the formation of H-complexes due to the influence of VWI only.
In (1) vgas,i is the frequency of O-H, N-H vibrations of i-th proton donor molecule in gas; vCCl4,i is the frequency of O-H, N-H vibrations of i-th proton donor molecule in neutral solvent CCl4; vH,i,j is the frequency of O-H, N-H vibrations of i-th proton donor molecule in H-complex with j-th proton acceptor molecule in neutral solvent. Thus, the value of H,i,j is a descriptor of the ratio of shifts in the frequencies of O-H and N-H vibrations of molecules of alcohols and azoles in a CCl4 medium relative to the gas phase in the presence and absence of H-complexes.
The value of the relative efficiency of H-bond formation βHi,j (ΔH) was determined from relation (2)
It should be noted that the value βH,i,j(ΔH) does not determine the true relative frequency shift of O-H or N-H vibrations due to H-bond formation alone, but shows the total shift due to both the influence of H-bond formation and the presence of VWI.
Nevertheless, in the literature, it is the value of H,i,j that is widely used both as a relative descriptor of the value of ΔH [2] and as a parameter for constructing correlation relations to determine the absolute values of ΔH [38].
Results and Discussion
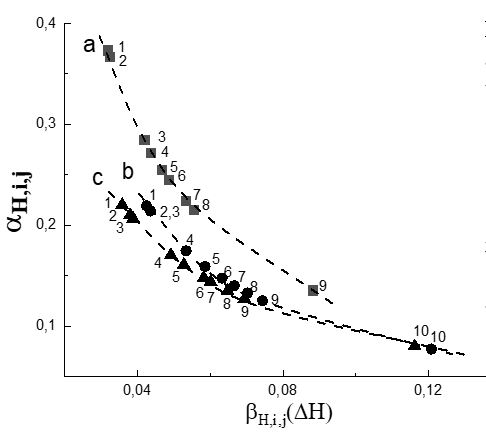
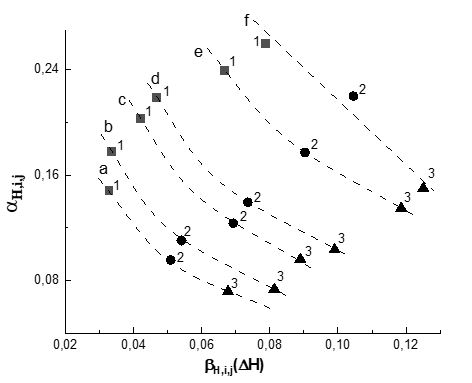
Fig. 1 shows the dependences of the values of α Н,i,j on the efficiency of H-bond formation βH,i,j(H) for H-complexes of alcohols {O-H...O, O-H...N}, and Fig. 2 shows the dependences of the values of α Н,i,j on the efficiency of H-bond formation βH,i,j(ΔH) for H-complexes of azoles {N-H...O}. It can be seen from the presented data that as the enthalpy of formation of H-complexes increases, the contribution of the frequency shift due to VWI to the overall shift decreases. This dependence means that the formation of H-bonds directly affects the electron density and, consequently, the force constant of O-H and N-H bonds much more effectively than the influence of the environment.
Figures 1 and 2 show that the value of α Н,i,j for H-complexes of methanol with acetonitrile and acetone (Fig. 1a1, Fig. 1a2), as well as H-complexes of benzimidazole and isatin with acetone (Fig. 2e1, Fig. 2f1) exceeds Size 0.25. Thus, if we conclude that for the above complexes the contribution to the shift in the frequencies of the O-H and N-H vibrations during the formation of the H-complex is due only to the presence of the H-bond, such a conclusion would not be completely correct.
The closeness of the dependence of the values of α Н,i,j on βH,i,j(ΔH) for the H-complexes of 2,2,3,3-tetrafluoro propan-l-ol and 2,2,2-trifluoro ethanol (Fig.1b, 1c) means that the efficiency of VWI of these alcohols with the environment is almost the same. This is apparently due to the fact that the values of α Н,i,j due to the VWI are determined by the individual nature of both proton-donor and proton-acceptor molecules, and since the structures and atomic composition of the molecules of these alcohols are very close, the values of α Н,i,j should not differ appreciably.
The obtained results allow us to make a cautious assumption that the absence of a strict linear relationship between the shift of the A-H vibrational frequency in the H-complex and the enthalpy of its formation ΔH, noted in [2,38], consists in the fact that the value of the enthalpy of formation was taken as a value proportional to the total shift of the A-H vibrational frequency relative to the gas (or neutral solvent) [38]), while a noticeable contribution to this shift may be given by the shift due to the influence of VWI. This circumstance may lead to the absence of a strict linear dependence between the shift of the A-H vibrational frequency in the H-complex and the enthalpy of its formation ΔH.
Also important for finding the true value of the enthalpy of formation may be the determination of the shift of the A-H vibrational frequency caused by VWI for H-complexes formed by weak H-bonds such as {N-H…π}, {O-H…π} {C-H…} {N-H…π} {S-H…π}{C-H…} [39, 40]. In such H-complexes, it is possible that the shift of the A-H frequency of the proton donor molecule due only to the influence of VWI may be comparable or even larger than the shift of the A-H frequency due directly to H-bond formation. Such a possibility was pointed out as early as in [40]. Thus, when using vibrational spectroscopy methods to obtain information on the enthalpy of H-bond formation, failure to take into account the contribution of VdW interactions may give incorrect values.
The application of the approach used in this work may allow us to directly identify the shift in the frequencies of the stretching vibrations of proton donor molecules caused by the VWI interaction in solutions with weak H-bonds. To do this, it is necessary to have information about the values of the vgas,i, vCCl4,i and vH,i,j (the frequency designations correspond to formula (2)) and then obtain the value of α Н,i,j from relation (2).
- WM Latimer, WH Rodebush (1920) Polarity And Ionization From The Standpoint Of The Lewis Theory Of Valence, J. Am. Chem. Soc. 42: 1419-25.
- J Pimentel, O McClellan (1964) Hydrogen bonding, Mir, Moscow.
- NG Bakhshiev (1972) Spectroscopy of intermolecular interactions, Nauka, Leningrad.
- ND Sokolov (1986) Van Der Waals Interaction and Hydrogen Bond Effects on Molecular Vibratonal frequency, Chem. Phys. 104: 371-81.
- OI Arkhangelskaya, NG Bakhshiev (1971) On the influence of Vanderwals intermolecular interactions on the infrared spectra of hydrogen bonded complexes. Opt. and Spectr. 27: 311-3.
- IH Reece, RL WERNER (1968) Intermolecular interaction in solution. The association of alcohols in solution and in the vapour phase. Spectrochimica Acta 24A: 1271-82.
- ND Sokolov (1981) Dynamics of hydrogen bonding. Infrared spectroscopy and spectral determination of hydrogen bonding energy. Nauka, Moscow. 63-88.
- OP Girin, NG Bakhshiev (1963) Influence of the solvent on the position and intensity of bands in infrared spectra of molecules. Uspekhi physicheskikh nauk 79: 235-40.
- G Pimentel, S Charles (1963) Infrared Spectral Perturbations In Matrix Experiments. Pure and Appl. Chim. 7: 111-4.
- AV Iogansen, BV Rassadin (1969) Dependence of amplification and displacement of infrared (OH) bands on hydrogen bonding energy. Journ . Prikl. spectroscopii 11: 1318-22.
- AS Wexler (1967) Integrated Intensities of Absorption Bands in Infrared Spectroscopy Appl. Spectr. Rev. 1: 29-98.
- J Kirkwood (1934) Theory of Solutions of Molecules Containing Widely Separated Charges with Special Application to Zwitterions. J. Chem. Phys. 2: 351-61.
- E Bauer, MJ Magat (1938) SUR LA DÉFORMATION DES MOLÉCULES EN PHASE CONDENSÉE ET LA LIAISON HYDROGÈNE. Phys. Etradium 9: 319-23.
- Y Hallam, T Rae (1961) Dielectric and dipolar interaction theories of solvent-induced infra-red frequency shifts. Nature 189: 915-6.
- A Pullin (1960) The influence of solvent on the position and intensity of bands in infrared spectra of molecules. Spectrochim. Acta 16: 12-4.
- E Hirota (1954) The Electrostatic Effect of the Solvents on the Frequency Shifts. Bull. Chem. Soc. Japan 27: 295-7.
- E Hirota (1953) On the Infrared Frequency Shift in the Liquid State and its Relation to the Atomic Polarization. Bull. Chem. Soc. Japan 26: 397-400.
- R Ulbrich (1968) Veränderungen charakteristischer Valenz-Frequenzen zweiatomiger organischer Gruppen beim Übergang vom Dampf in die flüssige Phase. Zs. Naturforsch. 23a: 1323-33.
- L Bellamy (1958) The origin of group frequency shifts-I Abnormal frequencies in multiple bonds. Spectrochim Acta 13: 60-3.
- L Bellamy, Y Hallam (1959) Infra-Red Spectra And Solvent Effects Part 3.-Intermolecular And Intramolecular Hydrogen Bonds. Trans. Farad, Soc. 55: 220-1.
- L Bellamy, D Williams (1960) Solvent effects on infra-red group frequencies. Pros. Roy. Soc. A 255: 22-25.
- AM Dolgonosov (2019) Representation of hydrogen bonding following from the theory of generalized charges. Zhurn. Strukt. Chem. 60: 1765-74.
- AN Egorochkin, OV Kuznetsov (2002) IR spectroscopy of hydrogen bonding and effects of substituents in electron-donor molecules. Izvestiya Akademii Nauk Chemical Series 5: 881-6.
- MA Varfolomeev, DI Abaidulina, AE Klimovitsky, BI Solomonov (2007) Influence of medium on the frequencies of vibrations of N-H and O-H groups of diphenylamine and phenol in complexes with different proton acceptors. Consideration of cooperativity. Journal of General Chemistry 77: 1677-83.
- IT Rakipov, FF Petrov, FF Akhmadiyarov, AA Khachatryan, AE Klimovskii, et al. (2019) FTIR spectroscopy of intermolecular interactions of pyrrole in solutions: The influence of media and cooperativity of Hydrogen bonds. J. Mol. Liq. 277: 200-6.
- RM Badger, SHJ Bauer (1937) Spectroscopic Studies of the Hydrogen Bond. II. The Shift of the O-H Vibrational Frequency in the Formation of the Hydrogen Bond. J. Chem Phys. 5: 839-52.
- AJ Stone (2017) Natural bond orbitals and the nature of hydrogen bonding. J. Phys. Chem. 121: 1531-4.
- CT Nemec, CJ Laconsaya, JM Calbrath (2018) Hydrogen bonding from a valence bond theory perspective: the role of covalency. PCCP 20: 20963.
- NIST/TRC Web Thermo Tables (WTT), NIST Standard Reference Subscription Database 3 - Professional Edition, Version 2-2012-1-Pro.
- CH Zhou, Y Wang (2012) Recent Researches in Triazole Compounds as Medicinal Drugs. Current Medicinal Chemistry 19: 239-80.
- Lee Seulki, Jun Lee Seung, Ahn Ahreum, Kim Yusic, Min Ahreum, et al. (2011) Infrared Spectroscopy of Imidazole Trimer in Helium Nanodroplets: Free stretch mode NH. Bull. Korean Chem. Soc., 32: 885-8.
- S Kumar, A Mukherjee, A Das (2012) Structure of Indole•••Imidazole Heterodimer in a Supersonic Jet: A Gas Phase Study on the Interaction between the Aromatic Side Chains of Tryptophan and Histidine Residues in Proteins. J. Phys. Chem. A. 116: 11573–80.
- PL José, MT Roque, RF Rosado, S Reva (2023) Dual Photochemistry of Benzimidazole. J. Org Chem. 88: 2884–97.
- A Westphal, MW Roth, D Spangenberg, Ch Janzen Schmitt (1999) The relative stabilities of benzotriazole tautomers determined by a rotational band contour analysis of the N–H stretching vibration. Chem. Phys. 248: 17–25.
- A Al-fahdawi, E Alsalihi (2018) Synthesis and Antibacterial Activity of Isatin Schiff Base Derivative with 3-Aminoacetophenone and its Ni II, Co II Transition Metals Complexes. ARO-THE SCIENTIFIC JOURNAL OF KOYA UNIVERSITY 10: 38-45.
- B.N. Narziev, N.U. Mulloev (1999) Proton-donor properties of heterocyclic compounds of pyrrole series according to IR spectroscopy. Zh. Strukt. Chem. 40: 585-9.
- Лаврик НЛ (2025) Influence of the Structure of Azole and Aliphatic Alcohol Molecules on the Effectiveness of Van der Waals Interactions with Carbon Tetrachloride. J. Org. Chem. Chem. Sci. 3б: 103
- VS Korobkov (1973) To the question of energy relations between the parameters of absorption bands of valence vibrations of CH groups and energies of hydrogen bonds. Journal of Applied Spectroscopy 19: 1125-7.
- VK Pogorelyi (1997) Weak hydrogen bonds. Uspekhi chemii XLVI: 602-38.
- VV Varfolomeeva, AV Terentyev (2017) Weak hydrogen bonds during adsorption of nonrigid molecules on graphitized carbon black. JSCH, 58: 586-613.

Figures at a glance