Diabetes Mellitus-Tuberculosis Comorbidity: A Literature Review
Received Date: April 11, 2023 Accepted Date: May 11, 2023 Published Date: May 15, 2023
doi: 10.17303/jomd.2022.2.101
Citation: Kakisingi C, Muteba M, Kabamba M, Tanon A, Situakibanza N-T H et al. (2023) Diabetes Mellitus-Tuberculosis Comorbidity: A Literature Review. J Obes Metab Dis 2: 1-19
Abstract
Tuberculosis-Diabetes mellitus co-morbidity is listed among the new challenges in TB control. Diabetes mellitus is recognized as a risk factor contributing to the development of TB disease and playing a deleterious role in the therapeutic outcome of patients undergoing TB treatment. Some of the pathophysiological mechanisms implicated in the occurrence of this unfavorable outcome during this comorbidity have been identified. This literature review allows us to highlight the role and impact of this co-morbidity so that clinicians and those responsible for programs to combat these two scourges are informed of its effects with a view to appropriate management of co-infected tuberculosis patients.
Keywords:Diabetes Mellitus; Tuberculosis; Comorbidity; Literature review
Introduction
Tuberculosis is the leading cause of infectious death in the world. It is caused by Mycobacterium tuberculosis (MTB), which is transmitted from person to person by aerosol droplets, usually through coughing.
In 2020, 10 million new cases of tuberculosis (TB) were reported worldwide with 1.5 million deaths [1]. In addition, it is estimated that one third of the world's population is latently infected with MTB[1]. The specific treatment of active tuberculosis includes multidrug therapy with Isoniazid, Rifampicin, Ethambutol, and Pyrazinamide, each of which has a specific mechanism of action. However, this anti-tuberculosis treatment is becoming increasingly problematic due to the emergence of strains of mycobacteria that are multi-drug resistant (MDR) to these antibiotics [1]. Therefore, many new therapeutic options are being explored as possible alternatives or adjunctive therapies to combat this devastating disease.
Despite considerable progress in the control of diabetes mellitus (DM), diabetic patients remain at high risk of contracting TB compared to non-diabetics [2-6]. In 2020, for a long time considered a condition of the wealthy "Western" countries of Europe and North America, it has been shown that type 2 diabetes mellitus (DM) is present, with high prevalence, even in the so-called resource-poor and middle-income countries. Indeed, there are now more people with diabetes in developing economies (Asia, Latin America and Africa) than in industrialised countries, and the proportion of diabetes cases related to insulin resistance is about 90-95% of the total prevalence of diabetes [7].
Glycated haemoglobin (HbA1c) is not only used as an indicator of plasma glucose level, but also utilised as a more reliable follow-up biomarker in that it shows the evolution of blood glucose in the previous months. However, the glucose overload test is instead used as a more reliable diagnostic parameter for type 2 diabetes mellitus than fasting blood glucose. Although more research is needed to establish the aetiology of type 2 diabetes, current research evidence indicates that genetic factors, obesity and possibly epigenetic (exosome) factors such as a high-fat or high-sugar diet may also be linked to the development of type 2 diabetes [8].
In addition, the complications linked to Type 2 DM are many and varied (vascular, nervous, metabolic, infectious, etc.). These represent major health problems. Although type 2 diabetes affects millions of people worldwide, studies on the epidemiology and co-morbidities associated with this scourge are largely limited by unspecific models of the disease that do not consider nutritional and polygenic factors involved in the development of diabetes mellitus in humans. Some authors acknowledge that diabetes decreases the efficiency of cell-mediated immunity, making diabetic patients more susceptible to Mycobacterium tuberculosis. However, the exact mechanism of Mycobacterium tuberculosis susceptibility in diabetics is not fully elucidated. Some animal studies have suggested that there may be a delay in immune recognition. A more recent study in humans suggests that epigenetic reprogramming may be responsible for increased inflammation, making individuals more vulnerable to infection (8). Nevertheless, research has shown that Mycobacterium tuberculosis infection may present differently in cases of co-morbid tuberculosis and diabetes mellitus.
Epidemiology
The burden of communicable diseases is particularly high among low-income countries. However, non-communicable diseases, which accounted for 47% of the burden of disease in 1990 in low-income countries, were expected to reach 69% by 2020 [9]. Increasing industrialisation and urbanisation, because of development and modernisation, and the nutritional transition, would lead to an increase in obesity and diabetes. The proportion of people with diabetes, which was 171 million in 2000, is expected to rise to 366-440 million by 2030, and three quarters of the projected diabetes patients are expected to be in low-income countries [10-12].
Diabetes carries a significant financial cost in resource-limited countries. For example, in Africa, where the average per capita health expenditure is US$ 30-800, the average annual cost of diabetes care is US$ 214-11,430 (direct costs US$ 876-1220) and this cost is usually borne by the patient due to the poorly organised health system [13]. In many countries, insulin is expensive or not widely available: a one-month supply of insulin can cost up to 20 days' salary [14]. Thus, social and economic conditions have a strong impact on treatment options [15]. In these resource-limited settings, TB continues to have high mortality and is among the top five causes of death [16]
TB, poverty and poor access to health services are closely linked, making TB management more difficult [17]. And associated co-morbidities such as diabetes mellitus further complicate the management of TB.
Several studies show that co-morbidity of tuberculosis and diabetes mellitus is common in both low and high income countries [18-21]. Recent studies have shown that DM prevalence among TB cases is variable. It ranges from about 50% to 2% in America, Asia, Europe, and Sub-Saharan Africa [22,23].
Pathophysiology
The interaction between Diabetes Mellitus and Tuberculosis
Diabetes as a risk factor of Tuberculosis
Diabetes is not only a risk factor for lower respiratory tract infections in general, but also particularly for TB. Although TB is more associated with other immunosuppressed conditions such as HIV infection, diabetes remains a significant risk factor in vulnerable populations due to its higher prevalence [24]. Stevenson et al. [25] reported that diabetes increased the risk of TB by 1.5 to 7.8 times, while Jeon and Murray [26], in a meta-analysis, reported that the relative risk of TB in patients with diabetes was 3.11
And in the latter study, the authors found that the prevalence of diabetes in TB patients ranged from 1.9% to 35% after screening, with the highest proportions found in regions of the world with the highest prevalence of diabetes [26]. Also, a United States study showed that the odds ratio of multidrug-resistant tuberculosis (MDR-TB) associated with diabetic patients is 2.1 [27]. Although type 2 diabetes is more common worldwide, the risk of TB in type 1 diabetes is three to five times higher [28,29] due to relatively poorer glycaemic control, lower body weight in type 1 diabetes patients and the younger age of type 1 diabetes patients [29]. Although it is not clear whether diabetes affects the clinical profile of TB, diabetic patients tend to have more lower lobe involvement compared to their non-diabetic counterparts and this could be explained by the reactivation of old foci [30]. In this regard, some studies have reported lower proportions of cavitated lung disease [27] while others have reported higher rates of cavitated lung disease [21,31,32].
Other authors have suggested that haemoptysis and fever are also common manifestations of co-morbid TB-DM. Atypical presentations have also been reported in relation to non-diabetic TB patients [25,27,33]. In relation to smear negativities, Alisjahbana et al. [27] reported in 2007 that diabetic TB patients had more symptoms, but no evidence of more severe disease and a lower proportion of smear negativities for acid-fast bacilli after two months of intensive TB treatment compared to their non-diabetic counterparts (18.1 % versus 10.0 %). However, these results were no longer statistically significant after adjusting for age, sex, BMI (body mass index), study site, chest X-ray abnormalities and sputum mycobacterial load prior to treatment initiation. The study also reported that after 6 months, 22.2% of sputum from diabetic patients still had Mycobacterium tuberculosis compared to 6.9% in controls (adjusted odds ratio: 7.65; p=0.004). A retrospective study of TB patients from southern Texas (USA) and north-eastern Mexico found that diabetic patients (identified by self-reporting) were more likely to remain positive at the first month (Texas cohort) or second month (Mexican cohort) of treatment [21]. Other studies have also shown a trend towards increased sputum conversion time (19,34–36), while others have shown no relationship between diabetes and sputum conversion rate at the end of the second month [19,37].
In relation to treatment outcome, diabetes would increase the risk of combined failure and death, death, and relapse in patients with TB. A systematic review by Baker et al. [38] reported that patients with diabetes had a hazard ratio (HR) for the combined outcome of failure and death of 1.69 (95% CI: 1.36 to 2.12), while the HR of death during TB treatment among 23 unadjusted studies was 1.89 (95% CI: 1.52 to 2.36). Diabetes was also associated with an increased risk of diabetes was also associated with an increased risk of relapse (RR: 3.89; 95% CI: 2.43 to 6.23). However, these authors did not find evidence of an increased risk of TB relapse with drug-resistant strains in people with diabetes [38]. In a Brazilian study to evaluate sociodemographic and clinical factors that may influence different TB outcomes in diabetic patients identified in the Brazilian National Health Directory between 2001 and 2011, it was found that the development of MDR TB was more related to relapse (OR = 9.60, 95% CI: 6.07-15.14), to a previous default (OR = 17.13, 95% CI: 9.58-30.63) and to a transfer of treatment centre (OR = 7.87, 95% CI: 4.74-13.07) [39]. Diabetes may affect the pharmacokinetics of anti-TB drugs, particularly rifampicin, by reducing their plasma concentrations [40]. However, there are conflicting reports on whether this affects the efficacy of TB treatment [30,40].
Tuberculosis as a Risk Factor for Diabetes
Although the association between diabetes and tuberculosis has long been recognized, studies to determine whether tuberculosis increases the risk of diabetes are few [41-43]. Tuberculosis can be responsible for impaired glucose tolerance (IGT) [43,44] and newly diagnosed diabetes [33,43]. Generally, IGT normalises after treatment of TB, but it is still an important risk factor for the development of type 2 diabetes later on [45]. For some authors, it is still unclear whether tuberculosis is a risk factor for newly discovered hyperglycaemia or diabetes in previously unscreened tuberculosis patients [42,43,46]. Basoglu et al [43], in a study of patients with active TB without a history of diabetes mellitus, found glucose intolerance in 10.4% and diabetes in 8.6% of the cohort; and compared to a matched control group of community acquired pneumonia, 17.4% were found to have diabetes and none had glucose intolerance. There was no significant difference between the two groups (p > 0.05). Oral glucose tolerance test (OGTT) results returned to normal in the TB and pneumonia groups after treatment. In a Nigerian study, a sequential follow-up of oral glucose tolerance tests in 54 patients with active TB, 42.6% of the patients examined showed pathological results, including 5.6% diabetes and 37.0% IGT(42). After three months on anti-TB therapy, only one of the eight patients with glucose intolerance on the second oral glucose tolerance test remained glucose intolerant, while only one patient was frankly diabetic. In a retrospective cohort study using data from the Oxford Record Linkage Study (ORLS) covering the period 1963 to 2005 in a predominantly white English population, Young et al. [41] found that although diabetes was associated with a two- to three-fold increased risk of TB, there was no evidence that TB increased the risk of diabetes. Thus, several literatures believe that hyperglycaemia, which may be related to TB infection and some commonly used anti-TB drugs, such as rifampicin and isoniazid, may have led to an overestimation of diabetes mellitus in previously unscreened TB patients [24,47,48]. However, tuberculosis infection as well as certain anti-tuberculosis drugs would have led to uncontrolled blood sugar levels in previously diagnosed diabetic patients [45]. This latter situation may therefore justify a re-evaluation of oral antidiabetic treatment or simply justify a switch to strict insulin therapy.
Underlying Pathophysiological Mechanisms
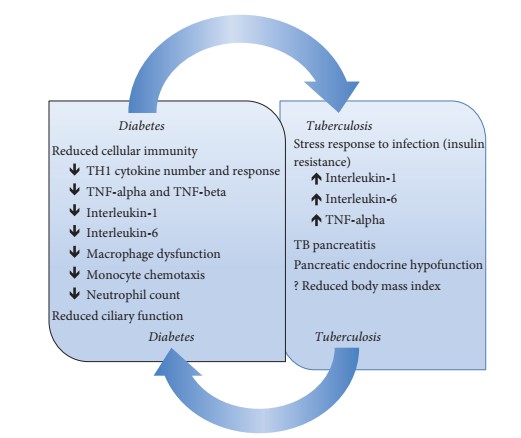
The increased risk of TB in diabetic patients is multifactorial [49-51] and several mechanisms have been suggested (see Figure 1). There is a decrease in cellular immunity due to a reduction in T-cell number and function and a low neutrophil count [49]. Diabetics have a reduced T-helper 1 (TH 1) cytokine response, tumour necrosis factor (TNF-alpha and TNF-beta), interleukin-1 and interleukin-6 compared to their non-diabetic counterparts [49,50]. The susceptibility of diabetic patients to tuberculosis is mainly due to reduced T-cell number and function, in particular the inhibition of TH1 cytokines from Mycobacterium tuberculosis, but also as a result of decreased IL-2 production There is macrophage dysfunction in diabetes resulting in impaired reactive oxygen species production, phagocytic and chemotactic functions [49,50].
Monocyte chemotaxis is also impaired in diabetic patients, a defect that does not improve with insulin [52]. Hyperglycaemia is also thought to impair the strength of the respiratory burst in expelling pathogens [49,50]. Although these proposed mechanisms are plausible, it is important that further studies are carried out to confirm them or not. The oxidative stress response to infection may also play a role in blood glucose disorders, a situation mediated by the effect of interleukin-1 (IL-1), interleukin-6 (IL-6) and TNF-alpha [24,45,53]. This temporal relationship has been demonstrated in some studies where it was found that between 19 and 42.6% of patients with active TB had GI or diabetes, with a significant reduction or complete regression of the prevalence after treatment [42,43]. In one of these studies, the rate of glucose intolerance was similar with the control group who had community-acquired pneumonia, supporting the possibility of a stress response to infection [43]. In contrast, in the study by Zack et al. [54], 41% of 256 patients admitted to the TB ward were glucose intolerant when oral glucose tolerance tests were performed after at least one month of hospitalisation and some of these developed frank diabetes. However, it is thought that the largely abnormal test results obtained one month after the start of treatment may reflect a true underlying glucose intolerance rather than a stress response to the infection [54]. Tuberculosis can also cause tuberculosis pancreatitis and pancreatic endocrine hypo-function, which can lead to de novo IGT or diabetes or worsen uncontrolled diabetes [45,53]. Tuberculous pancreatitis may not become evident until after the onset of diabetes [45,53]. Finally, malnutrition has been proposed in other studies as a risk factor for infections and glycaemic disorders. However, body mass index has not been associated with IGT or diabetes [33,42,43].
The role of Hyperglycaemic Stress
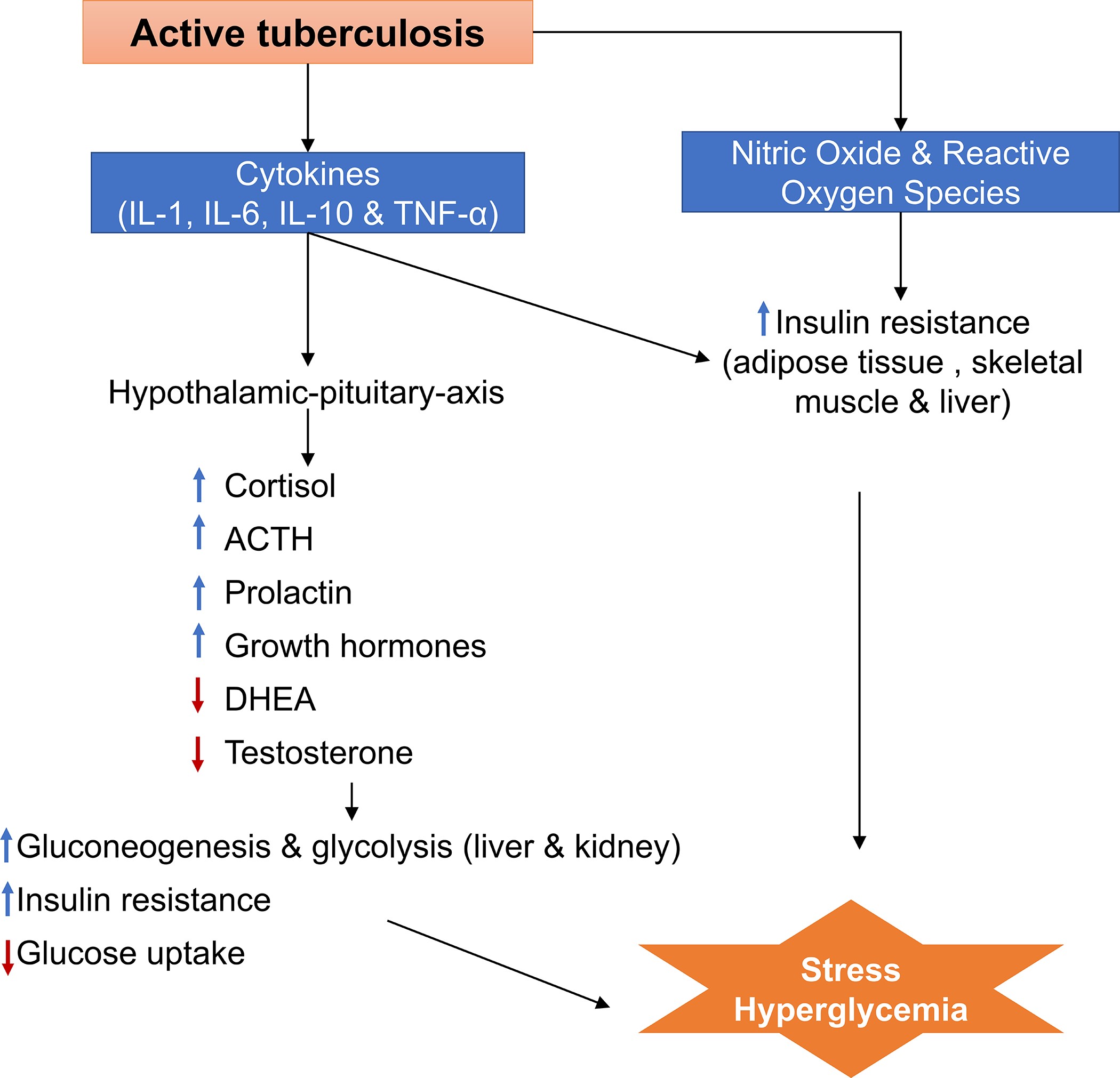
Stress hyperglycaemia is a state of blood glucose disturbance resulting from a variety of acute illnesses such as trauma, infection, surgery, or myocardial infarction, and usually resolves with resolution of the precipitating condition. Stress hyperglycaemia results from multiple signalling pathways, including counter-regulatory hormones such as glucagon, growth hormone, catecholamine and cortisol, and cytokines such as tumour necrosis factor-α (TNF-α) and the interleukins IL-1 and IL-6., metabolic and endocrine mechanisms during TB disease. In the absence of extensive studies on TB disease and stress-induced hyperglycaemia, we can assume that part of the hyperglycaemia in TB patients is due to acute stress resulting from changes in host immune, metabolic and endocrine mechanisms during TB disease (figure 2). During any infection, whether tuberculosis or other pathogens, pro-inflammatory cytokines and stress hormones can lead to stress hyperglycaemia by increasing hepatic glucose production (gluconeogenesis) and peripheral insulin resistance [56-59]. However, unlike other acute infections, the endocrine and immune responses in the context of TB disease are more prolonged in nature, as they can be activated during subclinical TB disease, symptomatic TB disease, during TB treatment, and even after TB treatment [60,61].
During initial infection with Mycobacterium tuberculosis (M.t.) and during TB disease, pro-inflammatory (IL-1, IL-6, interferon (IFN)-γ and tumour necrosis factor (TNF)-α) and anti-inflammatory (IL-10) cytokines are produced. In addition, macrophages generate nitric oxide and reactive oxygen species, T cells and natural killer (NK) cells [62,63]. In the context of obesity-related diabetes, increased pro-inflammatory cytokines, reactive oxygen species and nitric oxide cause insulin resistance through a cascade of inflammation pathways leading to hyperglycaemia [64–66]. As increased levels of pro-inflammatory cytokines, reactive oxygen species and nitric oxide are hallmarks of the host response to TB, similar mechanisms of hyperglycaemia may be involved in patients with active TB. The pro-inflammatory cytokines released during TB also activate the hypothalamic-pituitary axis (neuroendocrine pathway), increasing the release of cortisol, prolactin, oestradiol, catecholamines (dopamine, epinephrine, norepinephrine) and thyroid and growth hormones, while decreasing the production of dehydroepiandrosterone and testosterone [60,67-71].
A study conducted in South Africa by Opolot et al. [69] in hospital patients (n=160) found that, compared to patients with other acute stress conditions (n=89), patients with newly diagnosed smear-positive TB (n=71) had higher cortisol and dopamine levels than the non-tuberculosis group, and higher than normal levels of epinephrine and norepinephrine. Another study of newly diagnosed male pulmonary TB patients (n=30) found that growth and thyroid hormone, cortisol, oestradiol and prolactin levels were elevated and dehydroepiandrosterone and testosterone levels were lower than in the healthy male control group (n=19) [70]. As concentrations of catecholamines, cortisol, growth hormone, dopamine, epinephrine and norepinephrine are elevated during TB, it is plausible that these elevations result in increased gluconeogenesis and glycogenolysis in the liver and kidney and insulin resistance in peripheral tissues that may lead to subsequent hyperglycaemia [72-74]. In addition, the decrease in dehydroepiandrosterone and testosterone during TB correlates with insulin resistance, which may also indirectly increase the likelihood of hyperglycaemia [75-77].
Current studies on the effect of diabetes on TB treatment outcomes found that patients with diabetes had approximately twice the odds of death (pooled odds ratio [OR] 2.1, 95% confidence interval [CI] 1.8–2.5) and TB relapse (pooled OR 1.8, 95% CI 1.4–2.3) compared to patients without diabetes (78). However, most studies that estimated an association between diabetes and adverse TB outcomes did not stratify by previously diagnosed diabetes, undiagnosed pre-existing diabetes, or extent of hyperglycemia, and none have estimated the effect of stress hyperglycemia on TB treatment outcomes [78].
Clinical presentation of TB in TB-DM patients
Many studies suggest that DM is associated with the clinical presentation of TB. Patients with TB-DS comorbidity (compared to those without diabetes) are more likely to present with pulmonary, cavitary and microscopic positive TB at diagnosis. During TB treatment, TB-DM patients take longer to go from sputum smear positive to a negative sputum smear. Some studies also show that patients with DM are more likely to develop drug-resistant or multidrug-resistant TB, although this relationship is not observed in all series or cohorts in the literature [79].
In relation to the Anatomical site of the Disease
Pulmonary tuberculosis accounts for 70-80% of cases and it is generally acknowledged that immune compromise facilitates the haematogenous dissemination of M.t., predisposing to extra-pulmonary tuberculosis (EPT). This is the case for TB patients with HIV-AIDS [80] or those taking TNF inhibitors [81]. This is in contrast to patients with DST who are less likely to develop PET [3,21,39,82]. This may be due to a hyper-reactive cell-mediated immune response to M.t. in patients with DS that may be suboptimal for containing the growth of M.t. in the lung but sufficient to prevent its spread and reactivation elsewhere [83-85].
In Relation to Bacteriological Diagnosis
M.t. induces strong cell-mediated immunity leading to the formation of pulmonary granulomas (tubercles) which are considered a double-edged sword for the host [86]. The granulomas initially restrict the growth of M.t., but eventually, alas, it continues to replicate. These granulomas undergo caseation with rupture and spillage of thousands of viable bacilli into the respiratory into the respiratory tract. This "cavitary tuberculosis" explains the positivity of the sputum smears [87].
TB-DM patients are more likely than TB-non-DM patients to have TB with a higher sputum bacillary load [88,89]. Overall, a higher frequency of pulmonary versus extra-pulmonary TB, cavitary TB and a positive smear at diagnosis and extension during treatment would predict that TB-DM patients are more infectious than non-DM TB patients [90]. Such studies have not yet been described in our work setting.
In Relation to Drug Resistance
The relationship between drug-susceptible TB (DST) or multidrug-resistant TB (MDR-TB) and Creutzfeldt-Jakob disease (CJD) is unclear, with conflicting results on the association between higher drug-susceptible or multidrug-resistant TB in CJD patients compared to those without CJD [78,91–98]. However, these results are based on only four studies. Therefore, there is a need for more studies that would systematically assess the relationship between MDR-TB and diabetes comorbidity, by performing appropriate MDR-TB testing in the general population at the time of TB diagnosis (and not only in patients who have failed treatment), performing multivariate analysis to distinguish the independent contribution of diabetes from other confounding factors, and taking into account characteristics of the study population and local TB programme protocols, to understand how MDR-TB and diabetes may synergise [38].
Management of TB-DM comorbidity
Although it has been suggested that diabetes may lead to more severe TB disease, death and relapse, the dosage and duration of TB treatment do not differ between people with and without TB [38,99]. All screening and treatment centres (STCs) treat TB for six months for DST, consisting of an initial intensive phase of two months of rifampicin, isoniazid, pyrazinamide and ethambutol and a continuation phase of four months of rifampicin and isoniazid. For the neuro-meningeal form, among others, the treatment takes 12 months [99].
There is a proposed pharmacokinetic and pharmacodynamic interaction between anti-tuberculosis drugs and anti-diabetics. Rifampicin, which is a key component of the anti-tuberculosis cocktail, accelerates the metabolism of sulphonylureas and biguanides by enzymatic induction, thereby reducing their plasma levels, which may result in uncontrolled blood glucose levels [24,100]. In non-diabetics, it increases intestinal glucose absorption [99]. Isoniazid antagonises the action of sulphonylureas and worsens blood glucose control [48]. In some situations, isoniazid decreases the metabolism of oral antiglycaemic drugs and increases their plasma levels such as cytochrome P2C9 (CYP2C9) involved in the metabolism of sulphonylureas; however, the inducing effect of rifampicin is thought to far outweigh this inhibitory effect [101]. Similarly, it can inhibit insulin release, even in non-diabetics, causing hyperglycaemia [48]. Rifampicin and isoniazid are not known to significantly affect insulin degradation, as insulin is mainly degraded by hydrolysis of disulfide bonds by an insulin-degrading enzyme in the liver [102]. Theoretically, dipeptidyl peptidase (DPP) IV inhibitors can cause immunparesis and potentially worsen treatment outcomes in the management of TB [24,103]. Thiazolidinediones may be substrates for cytochrome P450 enzymes that are induced by rifampicin. Rosiglitazone is largely metabolised by CYP2C8 and rifampicin decreases rosiglitazone concentrations by 54-65% and pioglitazone by 54% [36]. The treatment of diabetes in tuberculosis infection requires careful evaluation and selection of anti-diabetic drugs. Again, the general approach to diabetes management does not differ according to the presence or absence of TB, despite the possible drug interactions described above [24,99].
Appropriate dietary advice is needed, taking into account the need for glycaemic control and the nutritional requirements of underweight or malnourished people [104]. Metformin remains the first-line antidiabetic agent, a relatively safe and inexpensive drug with a reduced incidence of hypoglycaemia [104]. Other agents to consider are sulphonylureas, meglitinides, alpha-glucosidase inhibitors, dipeptidyl peptidase (DPP) IV inhibitors, glucagon-like peptide (GLP) 1 analogues, thiazolidinediones and insulin. Obviously, in type 1 DM, insulin remains the essential treatment [104]. The choice of drug should be based on patient characteristics, availability, and adverse event profile. Indeed, treatment should be individualised [104]. Treatment can be intensified in terms of dose escalation or preference of a particular class or addition of one or more classes, depending on the situation, to achieve adequate glycaemic control or glycaemic targets [104].
In some situations, insulin is the preferred agent in type 2 diabetes in the presence of active TB infection [24]. The choice of insulin is justified by the severity of the TB infection, the loss of body tissue, the need for increased anabolism, hypofunction of the pancreas, the interaction between oral antidiabetic drugs and some anti-TB drugs, as noted above, and the possibility of associated liver disease that would preclude the use of oral agents [24,47]. For the above reasons, patients with pre-existing diabetes on oral therapy may be switched to insulin therapy if they have active TB once diagnosed, or if they are already on insulin; adjustments may be required if glycaemic control deteriorates. Once glucotoxicity has improved and infection is controlled, insulin requirements may decrease. However, requirements may increase again as appetite improves and food intake increases [24]. he choice of insulin should be based on safety, efficacy, cost, and patient characteristics. However, it should be noted that once the infection is controlled, oral antidiabetic drugs can be carefully considered [24]. Despite these advantages, insulin may or may not be readily available or expensive in some parts of the world [105]. For optimal control, regular monitoring of blood glucose levels is necessary. This allows early recognition of possible adverse effects, such as hypoglycaemia caused by some anti-diabetic drugs such as sulphonylureas and insulin, as well as assessment of the blood glucose profile that may require gradual dose adjustments. The fact that, in the majority of resource-limited countries, diabetic patients must be self-managed and that there is no compensation or reimbursement system, the achievement of the goals of correct disease monitoring and detection of acute complications related to DM, including hypoglycaemia, is hypothetical. However, patient education remains an essential part of patient management to understand the nature of the disease (TB and diabetes), the duration of treatment, adverse drug reactions and complications of the disease and to promote a healthy lifestyle [104-107].
Treatment of tuberculosis-diabetes mellitus comorbidity and outcome
Treatment of Tuberculosis-Diabetes Mellitus comorbidity
Treatment of tuberculosis in diabetic patients
The optimal treatment strategy for co-morbid TB and diabetes is not known. Diabetes is associated with an increased risk of TB treatment failure, death, and relapse, but it is not known whether optimal glycaemic control can partially or fully mitigate these negative effects, and whether TB treatment should be adapted in patients with diabetes. The treatment of drug-sensitive TB is highly standardised and usually consists of a 4-drug combination (rifampicin, isoniazid, pyrazinamide, and ethambutol) for the first 2 months of treatment and a combination of rifampicin and ethambutol for the remaining 4 months. In general, patients with this comorbidity are not treated differently from patients with tuberculosis alone. However, this approach should perhaps be reconsidered. It is unlikely that the increased failure rates of TB treatment in diabetic patients are due to high rates of TB drug resistance or poor adherence to treatment. Other causes of treatment failure could be more extensive tuberculosis, impaired immune response in people with diabetes (although this theory is more speculative), or reduced concentrations of anti-TB drugs in diabetic patients [108,109]. Another option to improve TB treatment outcomes in diabetic patients could be to extend the duration of treatment, a possibility that is advocated in some guidelines but has not yet been formally studied. However, the toxic effects of first-line anti-TB drugs, especially peripheral neuropathy with isoniazid and ocular toxicity with ethambutol, need to be considered as they may be more frequent due to, or in addition to, the complications of diabetes [110,111]. The dosage of ethambutol should be reduced when diabetic patients have reduced renal function [112].
Treatment of Diabetes in Patients with Tuberculosis
Optimal control of blood glucose levels could improve the outcome of TB treatment and prevent many of the complications associated with diabetes. However, TB often leads to a decrease in appetite, body weight and physical activity (patients may be tired and therefore less active), all of which can affect glucose homeostasis. Frequent monitoring is necessary to ensure good blood glucose control. Self-monitoring of blood glucose is not feasible in many settings and for some patients. Fasting blood glucose measurement in the clinic is not realistic but remains the best proposal for outpatient care in busy clinics in areas where TB is endemic and where random blood glucose and glycosuria measurements are less accurate than fasting blood glucose [113].
In the choice of drugs for diabetes, consideration should be given to possible drug interactions, especially for rifampicin, the most important anti-tuberculosis drug as it has reduced the duration of treatment for TB from 18 to 6 months, except for extra-pulmonary forms such as neuromeningeal TB for which treatment takes 12 months. Resistance to rifampicin is associated with poorer treatment outcomes, worse than for any other anti-TB drug. Rifampicin increases the hepatic metabolism of all sulphonylurea derivatives, the most widely used class of oral anti-diabetic drugs in the world. Less is known about the newer classes of anti-diabetic drugs, and studies are underway to demonstrate their contribution. Rifampicin probably has no effect on the exposure of glucagon-like peptide-1 receptor agonists and only a slight effect on dipeptidyl peptidase-4 inhibitors; however, the low availability and high cost of these drugs will limit their use in settings where TB is endemic [114].
Several other factors determine the choice of anti-diabetic drugs to be used in TB patients, such as availability, cost, ease of administration and safety. Safety issues include hypoglycaemia with sulphonylureas and insulin, lactic acidosis (especially under hypoxic conditions) with biguanides, gastrointestinal disturbances with biguanides, meglitinides and alpha-glucosidase inhibitors, and hypersensitivity to sulphonylureas (which may overlap with the adverse effects of TB drugs). The use of insulin at the start of TB treatment has been suggested; some national treatment guidelines (e.g.Indonesia) strongly suggest the use of insulin for diabetes in TB patients, although there is no evidence base to support this approach. As insulin is not metabolised in the liver, it does not have pharmacokinetic interactions with rifampicin or other anti-TB drugs, but insulin has several potential disadvantages when used in resource-poor settings, including cost, availability, storage and delivery [24].
Outcomes of Tuberculosis Treatment
Observational studies increasingly show that TB-DS co-morbidity is associated with increased adverse outcomes of TB treatment, including delays in achieving smear-negative results, treatment failures, deaths, relapses and reinfections [115-117]. The risk of recurrent TB disease after successful completion of treatment is also higher in those with DM compared with those who do not have DM [118].
In Relation to Sputum Smear Negativity and Treatment Failure
Patients with TB-DM comorbidity compared to TB-non-DM patients are more likely to remain sputum smear positive after the end of the intensive phase of treatment, and this result is an early predictor of treatment failure (sputum smear or culture positivity at five months or later during treatment), which is a major risk factor for the development of TB. This is also more likely to be the case in comorbid patients compared to TB-non-DM patients [38,117,119]. DM is most often associated with late smear negativity during treatment [116,120]. In other words, patients with this comorbidity compared to controls have a higher proportion of positive smears after the end of the intensive phase of treatment or have longer median days to sputum negativation. These findings are early predictors of treatment failure, which is also more common in patients with TB-DM comorbidity than in controls [38,117,119].
In Relation to Death
In the 1950s, studies reported that death was a common outcome in cases of comorbidity, and that these patients were more likely to die following a coma [121]. This finding was also reported by Root in 1934 after a study of 245 patients with this comorbidity [122]. In a systematic review and meta-analysis of the contemporary literature, Baker et al. [38] reported that the risk of death from tuberculosis or any other cause in 23 studies with unadjusted analyses was almost doubled (RR 1.89; 95% CI 1.52-2.36), and increased to 4.95 (95% CI 2.69-9-10) in 4 studies with analyses adjusted for age and potential confounders. This difference in results sufficiently demonstrates that it would be important to identify all confounding factors in TB-DM comorbidity studies.
In Relation to Relapse and Reinfection
TB-DM patients also appear to have a higher risk of TB disease. The observation by Baker et al. shows an almost fourfold increase in the risk of relapse in TB patients compared to non-DM TB patients (RR 3.89; 95% CI 2.43-6.23) [38]. A prospective study in southern Mexico of 1,262 TB patients further distinguished between relapse and reinfection and found higher adjusted probabilities of both outcomes in patients with DM compared to non-DM patients (OR= 1.8 for recurrence and relapse) [116].
Prevention of Tuberculosis-Diabetes Mellitus Comorbidity
Prevention of Tuberculosis
TB is a preventable bacterial infection and the prevention focuses on treating latent TB, early diagnosis, and vaccination [123].
Treatment of latent TB
Latent tuberculosis is an infection by the bacteria Mycobacterium tuberculosis where the individual affected does not have active infection or symptoms of tuberculosis infection. Individuals with latent tuberculosis infection (LTBI) remain asymptomatic and non-infectious until the bacteria become reactivated (Elekes et al.). The risk of developing active TB following LTBI is dependent on many factors, especially the immunological status of the infected host (American Thoracic). If effective chemoprophylaxis is given to individuals with LTBI, the risk of developing active TB is significantly reduced [124].
The treatment of latent tuberculosis infection (LTBI) is an essential component of tuberculosis (TB) elimination in regions that have a low incidence of TB. Four potentials drug regimens are recommended for the treatment of LTBI and there is considerable evidence for the risks and benefits of treatment:
isoniazid daily or semi-weekly for 9 months,
isoniazid daily or semi-weekly for 6 months,
isoniazid and rifapentine weekly for 3 months
or rifampin daily for 4 months.
These regimens are recommended only for the treatment of LTBI when the source organism is not suspected to be drug resistant [125].
Systematic Screening and Early Diagnosis of TB [126]
Systematic screening for TB disease is the systematic identification of people at risk for TB disease, in a predetermined target group, by assessing symptoms and using tests, examinations or other procedures that can be applied rapidly. Systematic screening can benefit people who are at risk getting TB, as early detection and start of treatment can improve their outcomes and reduce their costs.
The following groups should always be screened for TB:
- Household and close contacts of people with TB
- People living with HIV
- People in prisons and penitentiary institutions
- People exposed to silica (mainly miners)
- Patients with Diabetes Mellitus.
TB vaccination [127]
Vaccination is the most effective intervention for the control of infectious disease. The eradication of smallpox and rinderpest and the near eradication of polio have only been possible due to the availability of highly effective vaccines. While the BCG vaccine, first introduced in 1921, has been used worldwide to prevent life-threatening TB disease in infants and children, it has demonstrated limited and variable effectiveness in preventing pulmonary TB and the transmission of Mycobacterium tuberculosis (Mtb), the causal agent of TB, in adolescents and adults.
There are 13 vaccines currently in clinical development (self-reported by vaccine sponsors). These can be divided into:
whole cell-derived vaccines: The strategy of utilizing whole cell vaccines for TB has gained increased interest due to the ongoing difficulties in identifying individual antigens critical to generating protective immune responses to Mtb. Additionally, as whole organisms, these vaccines induce a more diversified immune response than do subunit-based vaccines, including both humoral and cellular immune responses to a range of protein, lipid and antigens. Currently candidates are M Vaccae, VPM1002, MTBVAC, MAR-901.
viral vectored subunit vaccines: Ad5Ag85A, ChAdOx185A, MVA85A and TB/FLU-04L are all viral vectors expressing antigen 85A (Ag85A) from Mtb. Ag85A is a mycolyl transferase enzyme important for cell wall synthesis. Ag85A is also involved in lipid accumulation and storage, potentially important in Mtb dormancy.
adjuvanted protein subunit vaccines: M72 + AS01E, H4 + IC31, H1 + IC31, H56 + IC31 and ID93 + GLA-SE are adjuvanted protein subunit vaccines. These vaccines are being developed as boosts to BCG to prevent de novo infection with Mtb and/or reactivation in those already infected.
Prevention of Diabetes Mellitus [128]
DM is at least theoretically preventable, and therefore, the most important goal of ultimately reducing the population burden of DM is to prevent the disease. Several methods that have been proven to be successful in prevention of DM include lifestyle modifications such as weight loss, increasing physical activity, and dietary changes.
Lifestyle modification has been difficult to maintain over a long term and has costs associated with regular visits to various health care professionals and lifestyle coaches. However, the WHO declared that: “supportive environments and communities are fundamental in shaping people’s choices, by making the choice of healthier foods and regular physical activity the easiest choice and therefore preventing overweight and obesity.”. At the individual level, people at risk for DM may limit energy intake from total fats and sugars, increase consumption of fruit and vegetables, as well as legumes, whole grains, and nuts, and engage in regular physical activity (60 min a day for children and 150 min spread through the week for adults).
An individual behavior change, which may prevent DM, needs to be facilitated by sustained implementation of evidence-based and population-based policies that make regular physical activity and healthier dietary choices available, affordable, and easily accessible to everyone.
- World Health Organization (2020) Global Tuberculosis Report 2020, Geneva.
- Lienhardt C, Fielding K, Sillah JS, Bât B, Gustafon P, Warndorff D et al. (2005) Investigation of the risk for tuberculosis: a case-control study in three countries. Int J Epidemiolology 34: 914-23.
- Ponce De Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernadez G et al. (2004) Tuberculosis and diabetes in southern Mexico. Diabete care 27: 1584-90.
- Pablos-Mendes A, Blustein J, Knirsh CA (1997) The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. Am J Public Health 87: 574-79.
- Alisjahbana A, Van Crevel R, Sahiratmadja E, Den Heijer M, Maya A, Istriana et al. (2006) Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis 10: 696-700.
- Perez A, Brown SH 3rd and Restrepo BI (2006) Association between tuberculosis and diabetes in the mexican border and non-border regions of Texas. Am J Trop Med Hyg 74: 604-11.
- Center for Disease Control (2020) National Diabetes Statistics Report.
- Prada-Medina CA, Fukutani KF, Kumar NP, Gil-Santana L, Babu S, Lichtenstein F et al. (2017) System Immunology of Diabetes-Tuberculosis comorbidity reveals signatures of diseases complications. Sci Rep 7: 1999.
- Boutayeb A (2006) The double burden of communicabe and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg 100: 191-9.
- L'Atlas du diabète de la FID 9ème ed. International Diabetes Federation, 2019.
- Wild S, Roglic G, Green A, Sicree and King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-53.
- King H, Aubert RE and Herman WH (1998) Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 21: 1414-31.
- Kirigia JM, Sambo HB, Sambo LG and Barry SP (2009) Economic burden of diabetes mellitus in the WHO African region. BMC Int Health Hum Rights 9: 6.
- Mendis S, Fukino K, Cameron A, Laing R, Filipe A, Khatib O et al. (2007) The availability and affordiability of selected essential medecines for chronic diseases in six-low and middle-income countries. Bull World Health Organ 85: 279-88.
- Sidibe EH (2000) Main complications of diabetes mellitus in Africa. Ann Med Interne 151: 624-8.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL (2006) Global and regional burden of disease and risk factors, 2001: sytematic analysis population health data. Lancet 367: 1747-57.
- Keshavjee S, Gelmanova IY, Pasecnhikov AD, Mishustin SP, Andreev YG, Yedilbayev A et al. (2008) Treating multidrug-resistant tuberculosis in Tomsk, Russia: developing programs thet adress the linkage between poverty and disease. Ann NY Acad Sci 1136: 1-11.
- Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C et al. (2007) Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health 7: 234.
- Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W (2009) Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 80: 634-39.
- Lindoso AA, Waldam EA, Komatsu NK, Figueiredo SM, Taniguchi M, Rodrigues LC (2008) Profile of tuberculosis patients progressing to death, city of Sao Paulo, Brazil, 2002. Rev Saude Publica 42: 805-12.
- Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith A et al. (2007) Type 2 diabetes an tuberculosis in a dynamic binational border population. Epidemiol Infect 135: 483-91.
- Eleke S, Lowe M, Kenny A, Clarke S, Eddershaw C, O’Drischoll M (2021) Screening, and treatment of latent tuberculosis: a systematic review of current evidence. TSLJ 21: 26-31.
- Workneh MH, Bjune GA, Yimer SA (2016) Prevalence and Associated Factors of Diabetes Mellitus among Tuberculosis Patients in South-Eastern Amhara Region, Ethiopia: A Cross Sectional Study. PLoS One 11: e0147621.
- Niazi AK, Kalra S (2012) Diabetes and tuberculosis: a review of the role of optimal glycemic control. J Diabetes Metab Disord 11: 28.
- Stevenson CR, Critchley JA, Forouhi NG, Roglic G, Williams BG, Dye C et al. (2007) Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn 3: 228-45.
- Jeon CY, Murray MB (2008) Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. Plos Med 5: e152.
- Alisjahbana B, Sahiratmadja E, Nelwan EJ, Purwa AM, Ahmad Y, Ottenhoff THM et al. (2007) The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis 45: 428-35.
- Feleke Y, Abdulkadir J, Aderaye G (1999) Prevalence and clinical features of tuberculosis in Ethiopan diabetic patients. East Afr Med J 76: 361-4.
- Swai AB, McLarty DG, Mugusi F (1990) Tuberculosis in diabetic patients in Tanzania. Trop Doct 20: 147-50.
- Wilson RM (1991) Infection and diabetes mellitus. In: Textbook of Diabetes. 1st ed. Oxford (UK): Blacwell Scientific Publications 813-9.
- Shaikh MA, Singla R, Khan NB, Sharif N, Saigh MO (2003) Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Med J 24: 278-81.
- Shaikh MA, Singla R, Khan NB, Sharif N, Saigh MO (2003) Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Med J 24: 278-81.
- Mugusi F, Swai AB, Alberti KG, Mclarty DG (1990) Increased prevalence of diabetes mellitus in patients with pulmonary tuberculosis in Tanzania. Tubercle 71: 271-6.
- Heysell SK, Moore JL, Keller SH, Houpt (2010) Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis 16: 1546-53.
- Chang JT, Dou HY, Yen CL, Wu YH, Huang RM, Lin HJ et al. (2011) Effect of type 2 diabetes mellitus on the clinical severity and treatment outcome in patients with pulmonary: a potentiel role in the emergence of multidrug resistance. J Formos Med Assoc 110: 372-81.
- Dooley KE, Chaisson RE (2009) Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 9: 737-46.
- Kameda K, Kawabata S, Masuda (1990) Follow-up study of short course chemotherapy of pulmonary tuberculosis complicated with diabetes mellitus. Kekkaku 65: 791-803.
- Baker MA, Harries AD, Jeon CY, Hart JE, Kapur A, Lönnroth K et al. (2011) The impact of diabetes on tuberculosis treatment: a systematic review. BMC Med 9: 81.
- Reis-Santos B, Gomes T, Locatelli R, De Oliviera E, Sanchez MN, Horta BL et al. (2014) Treatment outcomes in tuberculosis patients with diabetes: a polytomous analysis using Brazilian surveillance system. PloS One 9: e100082
- Baghaei P, Marjani M, Javanmard P, Tabarsi P, Masjedi MR (2013) Diabetes mellitus and tuberculosis facts and controversies. J Diabetes Metab Disord 12: 58.
- Young F, Wotton CJ, Critchley JA, Unwin NC, Goldacre. Increased risk of tuberculosis disease in people with diabetes mellitus: record-linkage study in a UK population. J Epidemiol Community Health 66: 519-23.
- Oluboyo PO, Erasmus RT (1990) The significance of glucose intolerance in pulmonary tuberculosis. Tubercle 71: 135-8.
- Basoglu OK, Bacakoglu F, Cok G, Sayiner A, Ates (1999) The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis 54: 307-10.
- Jeon CY, Harries AD, Baker MA, Hart JE, Kapur A, Lönroth K et al. (2010) Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Health 15: 1300-14.
- Guptan A, Shah A (2000) Tuberculosis and diabetes: an appraisal. Indian Journal of Tuberculosis 47: 3-8.
- Bansal V, Asmar NE, Selman WR, Arafah BM (2015) Pitfalls un the diagnosis and managemen of Cushing's syndrome. Neurosurg Focus 38: E4.
- Atkin SL, Masson EA, Bodmer CW, Walker BA, White MC (1993) Increased insulin requirement in a patient with type 1 diabetes on rifampicin. Diabet Med 10: 392.
- Lebovitz HE (1988) Oral hypoglycemic agents. Prim Care 15: 353-69.
- Geerlings SE, Hoepelman (1999) Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol 26: 259-65.
- Tsukaguchi K, Yoneda T, Yoshikawa M, Tokuyama T, Fu A, Tomoda et al. (1992) Case study of interleukin-1 beta, tumor necrosis factor alpha and interleukin-6 production peripheral blood monocytes in patients with diabetes mellitus complicated by pulmonary tuberculosis. Kekkaku 67: 755-60.
- Sidibe EH et al (2007) Diabete et tuberculose pulmonaire : aspects épidémiologiques, physiopathologiques et symptomatologiques. Cahiers d'études et de recherches francophones /Santé 17: 29-32.
- Moutschen MP, Scheen AJ, Lefebvre PJ (1992) Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab 18: 187-201.
- Geevarghese PJ (1968) A clinicopathological study of growth onset diabetes with pancreatic calculi. Pancreatic diabetes.
- Zack MB, Fulkerson LL, Stein E (1973) Glucose intolerance in pulmonary tuberculosis. Am Rev Respir Dis 108: 1164-9.
- Yorke E, Atiase Y, Akpalu J, Sarfo-Kantanka O, Boima V, Dey ID (2017) The bidirectional relationship between Tuberculosis and Diabetes. Tuberculosis research and treatment 1702578.
- Dugan KM, Braithwaite SS, Preiser JC (2009) Stress hyperglycemia. Lancet 373: 1798-807.
- McCowen KC, Malhotra A, Bristian BR (2001) Stress-induced hyperglycemia. Crit Care Clin 17: 107-24.
- Marik PE, Raghavan M (2004) Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med 30: 748-56.
- McGuiness O (2005) Defective glucose homeostasis during infection. Annu Rev Nutr 25: 9-35.
- Kleynhans L, Ruzive S, Ehlers L, Thiart L, Chegou NN, Conradie et al. (2017) Changes in host immune-endocrine relationships during tuberculosis treatment in patients with cured and failed treatment outcomes. Front Immunol 8: 690.
- Malherbe ST, Shenai S, Ronacler K, Loxton AG, Dolganov G Kriel et al. (2016) Persisting positron emission lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 22: 1094-100.
- Lerner TR, Borel S, Gutierrez MG (2015) The innate immune response in humane tuberculosis. Cell Microbiol 17: 1277-85.
- Stutz MD, Clark MP, Doerflinger M, Pellegreni M (2018) Mycobacterium tuberculosis: Rewiring host cell signaling to promote infection. J Leukoc Biol 103: 259-68.
- Wieser V, Moschen AR, Tilg H (2013) Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz) 61: 119-25.
- Wellen KE, Hotamosligil GS (2005) Inflammation, stress and diabetes. J Clin Invest 115: 1111-9.
- De Luca C, Olefsky JM (2008) Inflammation and insulin resistance. FEBS lett 682: 97-105.
- Bottasso O, Bay ML, Besedovsky H, Del Rey A (2009) Immunoendocrine altérations during human tuberculosis as an integrated view of disease pathology. Neuroimmunomodulation 16: 68-77.
- Besedovsky HO, Del Rey A, Klusman I, Furkawa H, Arditi GM, Kabiersch (1991) Cytokines as modulators of the hypothalamus-pituitary-adrela axis. J steriod Biochem Mol Biol 40: 613-8.
- Opolot JO, Theron AJ, Anderson R, Feldman C (2015) Acute phase proteins and stress hormone responses in patients newly diagnosed active pulmonary tuberculosis. Lung 193: 13-18.
- Del Rey A, Lahuad CV, Bozza VV, Bogue C, Farroni MA, Bay M et al. (2007) Endocrine and cytokine responses in humans with pulmonary tuberculosis. Brain Behav Immunol 21: 171-9.
- Bottasso O, Bay ML, Besedovsky, Del Rey A (2007) The immuno-endocrine component in the pathogenesis of tuberculosis. Scand J Immunol 66: 166-75.
- Kim SH, Park MJ (2017) Effects of growth hormone glucose metabolism and insulin resitance in human. Ann Pediatr Endocrinol Metab 22: 145-52.
- Xiu F, Stanojcic M, Diao, Jeschke MG (2014) Stress hyperglycemia, insulin treatment, and innate immune celles. Int J Endocrinol 486403.
- Rubi B, Maechler P (2010) Mini review: new roles for peripheral dopamine on metabolic control and tumor growth let's seek the balance. Endocrinology 151: 5570-81.
- Li Y, Zhang M, Liu X, Cui W, Rampersad S, Li F et al. (2017) Correlates and prevalence of hypogonadism in patients with early and late onset type 2 diabetes. Andrology 5: 739-43.
- Li C, Ford ES, Li B, Giles WH, Liu S (2010) Association of testosterone and sex hormone-binding globulin with metabolic syndrom and insulin resistance in men. Diabetes Care 33: 1618-24.
- Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB (2008) Inverse association of testosterone and the metabolic syndrome in men is cosisttent across race and ethnic groups. J Clin Endocrinol Metab 93: 3403-10.
- Magee MJ, Kempker RR, Kipiani M, Gandhi NR, Darchia L, Tukvadze N et al. (2015) Diabetes mellitus is associated with cavities, smear grade, and multidrug-resitant tuberculosis in Georgia. Int J Tuberc Lung Dis 19: 685-92.
- Restrepo BI, Schlesinger LS (2014) Impact of diabetes on the natural history of tuberculosis. Diabetes Res Clin Pract 106: 191-99.
- Leeds IL, Magge MJ, Kurbatova EV, Del Rio C, Blumberg HM, Leonard MK et al. (2012) Site of extrapulmonary tuberculosis is associated with HIV infection. Clin Infects Dis 55: 75-81.
- Harris J, Keane J (2010) How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol 161: 1-9.
- Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung GM et al. (2008) Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol 167: 1486-94.
- Restrepo BI, Fisher-Hoch SP, Pino PA, Salinas A, Rahbar MH, Mora F et al. (2008) Tuberculosis in poorly controlled type 2 diabetes; altered cytokine expression in peripheral blood cells. Clin Infect Dis 47: 634-41.
- Walsh MC, Camerlin AJ, Miles R, Pino P, Martinez P, Mora-Guzman F et al. (2011) The sensitivity of interferon-gamma release assays is not compromised in tuberculosis patients with diabetes. Int J tuberc Lung Dis 15: 179-84.
- Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S (2013) Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincent type 2 diabetes mellitus. J Infect Dis 208: 739-48.
- Guirado E, Schesinger LS, Kaplan G (2013) Macrophages in tuberculosis: friend or foe. Semin Immunol 35: 563-83.
- Russell DG (2007) Who puts the tubercle in tuberculosis? Nat Rev Microbiol 5: 39-47.
- Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R et al. (2012) Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS ONE 7: e41367.
- Restrepo BI (2007) Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin Infect Dis 45: 436-38.
- Behr MA, Warren SA, Hopewell PC, Ponce de Leon A, Daley CL, Small PM (1999) Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast-bacilli. Lancet 353: 444-9.
- Abdelbary BE, Garcia-Viveros M, Ramirez-Oropesa H, Rahbar MH, Restrepo BI (2016) Transmission-diabetes epidemiology in the border and non-border regions of Tamaulipas, Mexico. Tuberculosis (Ednib) 101: 124-34.
- Perez-Navarro LM, Fuentes-Dominguez FJ (2015) Zenteno-Cuevas. Type 2 diabetes mellitus and its influence in the development of multidrug resistance tuberculosis in patients from southeastern Mexico. Journal of Diabetes and its Complications 29: 77-82.
- Bashar M, Alcabes P, Rom WN, Condos R (2001) Increased incidence of multidrug-resitant tuberculosis in diabetic patients on the Bellevue Chest Service, 1987 to 1997. Chest 120: 1514-9.
- Wanga PD, Lin RS (2001) Drug-resistant tuberculosis in Taipei, 1996-1999. Am J Infect Control 29: 41-7.
- Subhash HS, Ashwin I, Mukundan U, Danda D, John G, Cherian AM et al. (2003) Drug resistant tuberculosis in diabetes mellitus: a retrospective study from south India. Trop Doct 33: 154-6.
- Hsu AH, Lee JJ, Chiang CY, Li YH, Chen LK, Lin CB (2012) Diabetes is associated with drug-resistant tuberculosis in Eastern Taiwan. Int J Tuberc Lung Dis 17: 354-56.
- Gomez-Gomez A, Magana-Aquino M, Lopez-Meza S, Aranda-Alvarez M, Diaz-Ornelas DE, Hernandez-Segura MG et al. (2015) Diabetes and other risk factors for multi-drug resistant tuberculosis in a mexican population with pulmonary tuberculosis: cas control study. Arch Med Res 46: 142-8.
- Fisher-Hoch SP, Whitney E, McCormick JB, Crespo G, Smith B, Rahbar MH et al. (2008) Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis 40: 888-93.
- World Health Organization. Global Tuberculosis Report. 2016.
- Aguiree F, Brown A, Cho NH, Dodd S, Dunning T, Hwang M et al. (2013) IDF Diabetes Altals 6th edition. International Diabetes Federation.
- Venkatesan K (1992) Pharmacokinetic drug interactions with rifampicin. Clin Pharmacokinet 22: 47-65.
- Duckworth WC, Bennett RG, Hamel FG (1998) Insulin degradation: progress and potential. Endocr Rev 19: 608-24.
- Mabsbad S (2009) Treatment of type 2 diabetes with incretin-based therapies. The Lancet 373: 438-39.
- American Diabetes A (2016) Standards of Medical Care in Diabetes 2016 Abridged for Primary Care Providers. Clinical Diabetes 34: 3-21.
- Beran D, Ewen M, Laing R (2016) Constraints and challenges in access to insulin: a global perspective. The Lancet Diabetes & Endocrinology 4: 275-85.
- Riza AL, Pearson F, Ugarte-Gil C, Alisjahbana B, Van de Vijver S, Panduru NM et al. (2014) Clinical management of concurrent diabetes and tuberculosis and the implications for patients service. The Lancet Diabetes & Endocrinology 2: 740-53.
- World Health Organization. Collaborative framework for care and control of tuberculosis and diabetes, 2011.
- Ruslami R, Aarnouste RE, Alisjahbana B, Van der Ven AJ, Van Crevel R. (2010) Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health 15: 1289-99.
- Chiang CY, Lee JJ, Chien ST, Enarson DA, Chang YC, Chen YT et al. (2014) Glycemic control and radigraphic manifestations of tuberculosis in diabetic patients. PLoS ONE 9: e93397.
- Van Ingen J, Aarnouste RE, Donald PR, Diacon AH, Dawson R, Van Balen GP et al. (2011) Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 52: e194-9.
- Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, Van der Ven AJ et al. (2013) Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 13: 27-35.
- Waugh N, Scotland G, McNamee P, Gillet M, Brennan A, Goyder et al. (2007) Screening for type 2 diabetes: literature review and economic modelling. Health Techol Assess 11: 1-125.
- Rotchford AP, Rotchford KM, Machattie T, Gill GV (2002) Assessing diabetic control-reliability of methods available in resource poor settings. Diabet Met 19: 195-200.
- Tornio A, Niemi M, Neuvonen PJ, Backman JT (2012) Drug interactions with oral antidiabetics agents: pharmcokinetic mechanisms and clinical implications. Trends Pharmacol Sci 33: 312-22.
- Baker MA, Lin HH, Chang HY, Murray MB (2012) The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis 54: 818-25.
- Jeon CY, Murray MB an Baker MA (2012) Managing tuberculosis in patients with diabetes mellitus: why we care and what we know. Expert Rev Anti Infect Ther 10: 863-8.
- Jimenez-Corona ME, Cruz-Hervert LP, Garcia-Garcia L, Ferreyra-Reyes L, Delagado-Sanchez G, Bodadilla-Del-Valle M et al. (2013) Association of diabetes and tuberculosis: impact on treatment and post-treatment outcomes. Thorax 68: 214-20.
- Viswanathan V, Vigneswari A, Selvan K, Satayavani K, Rajeswari R, Kapur A (2014) Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis: a report from South India. J diabetes Complications 28: 162-5.
- Jorgensen ME, Faurholt-Jespen D (2014) Is there an effect of glucose lowering treatment on incidence and prognosis of tuberculosis? A systematic review. Curr Diab Rep 14: 505.
- Silwer H, Oscarsson (1958) Incidence and coincidence of diabetes mellitus and pulmonary tuberculosis in a Swedish county. Acta Med Scand Suppl 1-48.
- Morton R (1964) Phthisiologia, or, a treatise of consumptions. London.
- Root HF (1934) The association of diabetes and tuberculosis. N Eng J Med 210: 1-13.
- World Health Organization. Global Tuberculosis Report. 2022.
- Lobue P, Menzis D (2010) Treatment of latent tuberculosis infcetion: an update. Respiral Carton Vic 15: 603-22.
- Tang P, Johnston J (2017) Treatment latent tuberculosis infection. Curr Treat Options Infect Dis 9: 371-9
- World Health Organization. Consolitated guidelines on tuberculosis. 2022.
- Fletcher H, Schrager L (2011) Tuberculosis vaccine development and the End TB strategy: importance and current status. Trans R Soc Trop Med Hyg 110: 212-8.
- Blüher M, Stumvoli M (2018) Diabetes complications, comorbidities and related factors. Springer Nature 1-24

Figures at a glance