Effect of Mushroom Tea Formulations Combined with Ginger or Lemongrass on Anti-Obesity Effects In Vitro
Received Date: January 16, 2025 Accepted Date: February 16, 2025 Published Date: February 19, 2025
doi:10.17303/jomd.2025.2.101
Citation: Chieguen Tchieguen Inès, Dongmo Alain Bertrand, Behoroum Allayanan Eveil, Tendja Kwatchou Raina Imel, Tetakounte Madoum Laurine Naëla, et al. (2025) Effect of Mushroom Tea Formulations Combined with Ginger or Lemongrass on Anti-Obesity Effects In Vitro. J Obes Metab Dis 2: 1-18
Abstract
Obesity is a public health problem due to its increasing prevalence and the complications it causes. The aim of this study was to formulate herbal teas based on a mixture of three oyster mushroom species combined with ginger or lemongrass and to assess their anti-obesity properties in vitro. Firstly, individual herbal teas of mixtures of mushrooms, lemongrass and ginger were prepared by infusing 3 g of dry matter in 150 ml of water, in order to assess their antioxidant (FRAP and DPPH tests), anti-inflammatory, digestive enzyme (pancreatic α-amylase, α-glucosidase and pancreatic lipase) and anti-inflammatory activities in vitro. Secondly, in order to optimise the properties of the mushroom teas, formulations combining mushrooms with ginger or lemongrass were developed using random mixing, with the proportion of mushrooms varying between 70 and 80 %. The parameters previously assessed were repeated. The results showed that, among the individual teas, the ginger and lemongrass teas were the most active, with the exception of the lipase inhibition test, where the mushroom tea was the most active. Ten herbal teas were developed, including five made from mushrooms and ginger (G1-G5) and five others from mushrooms and lemongrass (C1-C5). All Ten herbal teas exceeded the efficacy of mushroom-based teas alone. Moreover, the best formulations for each group were C1 (2.1g mushrooms + 0.9g ginger) and G1 (2.1g mushrooms + 0.9g lemongrass), with C1 showing higher levels of activity than G1 and sometimes comparable or even higher than the standards. Mushroom teas combined with ginger or lemongrass could be an alternative in the fight against obesity.
Keywords: Obesity; Oyster Mushrooms; Ginger; Lemongrass; Herbal Tea
Introduction
Obesity is a public health problem, resulting in the abnormal and excessive accumulation of fat in the body, which can damage health [1]. Obesity is a pathology resulting from a chronic imbalance between calorie intake and energy expenditure, where excess energy is stored in the form of triglycerides in adipose tissue. According to the WHO, more than 1.9 billion adults were overweight, of whom 650 million were obese, representing 13% of the world's adult population; this rate is projected to rise to 20% by 2025 [2]. In Cameroon, the prevalence of obesity rose from 5.6% in 2005 to 9.6% in 2016 [3]. This growing prevalence highlights awareness of the true scale of this epidemic. Obesity is caused by a number of factors, including a sedentary lifestyle and the consumption of high-calorie foods (foods rich in sugars and fats), which are primary contributors, genetic factors, the use of certain drugs, insulin resistance and oxidative stress [4].
Given its complexity and complications, obesity is of particular interest because, if left untreated, it leads to a succession of metabolic disorders resulting in the onset of numerous pathologies such as diabetes, hypertension, hyperlipidaemia, cardiovascular disease, gout, respiratory genes, cancer, arthritis and infertility [1,2].
In addition, the WHO recommends weight reduction as a treatment for obesity, by reducing energy intake through a healthy, balanced diet and physical activity [2]. Furthermore, because of the metabolic disorders it causes, notably hyperglycaemia, hyperlipidaemia, oxidative stress, inflammation and many others, several therapeutic targets need to be addressed in the treatment of obesity, namely the use of antioxidants, digestive enzyme inhibitors, anti-inflammatories and satiety inhibitors [1]. The use of antioxidants could reduce the oxidative stress induced by obesity. Similarly, inhibitors of digestive enzymes (alpha amylase, alpha glucosidase and lipase) are thought to reduce intestinal absorption of nutrients (glucose, fatty acids and glycerol), leading to a reduction in weight. Anti-inflammatories inhibit the action of pro-inflammatory mediators and enzymes involved in adipose tissue inflammation and oxidative stress. Satiety inhibitors, on the other hand, are thought to reduce food intake [1,5]. These different therapeutic targets have already been demonstrated in several functional foods, which have the advantage of being available to most of the population with fewer side effects than pharmacotherapy.
Hence the search for oyster mushrooms, namely: Pleurotus floridanus, Pleurotus sajor-cajou, Pleurotus pulmonarius, the rhizomes of Zingiber officinale (ginger) and the leaves of Cymbopogon citratus (lemongrass), which stand out for their wealth of bioactive metabolites (polyphenols, flavonoids, tannins, glycosides, anthocyanins, etc.) and their established therapeutic properties [6-8]. Several studies on oyster mushrooms have demonstrated their antioxidant, anti-diabetic, antihypertensive, hepatorotective and renoprotective properties [9-11]. It has also been reported that lemongrass and ginger possess antioxidant, anti-inflammatory and digestive enzyme inhibitory properties in vitro and are used in vivo in the management of numerous metabolic diseases such as diabetes, obesity and many others [7,12].
In addition to being functional foods, oyster mushrooms, ginger and lemongrass offer nutritional composition suitable for a low-calorie diet.
In traditional medicine, functional foods are generally consumed in the form of herbal teas. Herbal teas are defined as drinks obtained by decoction, infusion or maceration of plant material in cold or hot water. They have the advantage of being easy to prepare and effective in extracting bioactive compounds quickly and easily [13].
Nevertheless, the use of mushroom teas in the fight against obesity remain largely unknown to the general public, unlike ginger or lemongrass herbal teas.
Previous work from which this study was derived showed that the mixture of mushrooms (Pleurotus floridanus, Pleurotus sajor-cajou, Pleurotus pulmonarius) at a ratio of 1:1:1 possessed greater antioxidant and digestive enzyme inhibiting activity than the mushrooms taken individually [10,14]. In addition, the addition of 3 % lemongrass to this mixture of mushrooms via a herbal tea increased the antioxidant activity of the latter and, finally, the aqueous extracts of ginger and lemongrass had an antioxidant activity superior to that of the mushrooms.
In view of the above, and with a view to boosting the activities of mushroom teas and using them to combat obesity, the aim of this study was to formulate teas based on a mixture of oyster mushrooms supplemented with ginger or lemongrass and to assess their anti-obesity effects in vitro.
Materials and Methods
Plant Material
The fresh mushrooms Pleurotus floridanus, Pleurotus sajor-cajou and Pleurotus pulmonarius were harvested in Douala at the mushroom farm of the Faculty of Sciences of the University of Douala. The rhizomes of Zingiber officinale (ginger) of the yellow variety and the leaves of Cymbopogon citratus (lemongrass) were collected from a farm at Missoke in the town of Douala (Littoral Cameroon), known for its rigorous production practices and whose products are regularly sold on the local market.
Cleaning, Drying and Grinding
The fresh mushrooms, ginger rhizomes and lemongrass leaves were cleaned, cut up and dried in an oven (BLINDER brand) at 35°C for 72 hours. They were then ground in a blender to obtain fine powders. The powders from the three mushrooms were mixed in a 1:1:1 ratio (Pleurotus floridanus, Pleurotus sajor-cajou and Pleurotus pulmonarius). Subsequently, the powder mixtures of three mushrooms, ginger and lemongrass were stored individually in dry glass jars kept in the dark for further testing.
Before the herbal teas were formulated, individual mushroom, lemongrass and ginger teas were prepared in order to evaluate their effects on certain anti-obesity physiological aspects in vitro.
Preparation of Mixed Mushroom, Lemongrass and Ginger Herbal Teas and Evaluation of their Effects on some Anti-obesity Physiological Aspects
To do this, 3 g of mixed oyster mushroom powders, lemongrass powders and ginger powders were packaged in teabags and percolated in 150 mL of hot water for 15 minutes. The three herbal teas were then subjected to quantitative phytochemical screening (polyphenols, flavonoids and tannins), antioxidant tests (DPPH and FRAP), digestive enzyme inhibition tests (pancreatic alpha amylase, alpha glucosidase and pancreatic lipase) and the anti-inflammatory test by assessing the inhibition of egg albumin denaturation.
Quantitative Phytochemical Screening
Determination of Polyphenols
The total phenolic content was estimated by colorimetry using the Folin-Ciocalteu procedure and gallic acid as the reference molecule. Briefly, from the extracts, a 1 mg/ml aqueous solution was prepared for this analysis, while the reaction mixtures were prepared by taking 0.5 ml of the extract solution, 2.5 ml of the 10% Folin-Ciocalteu test agent dissolved in water and 2.5 ml of the 7.5% NaHCO3 aqueous solution. The mixture was then incubated for 45 minutes. The absorbance was obtained using a spectrophotometer at a wavelength of 765 nm. The same method was repeated for gallic acid and then the required calibration curve was constructed. The results were expressed as gallic acid equivalent per mg extract (GAEq/mg) for each sample [15].
Flavonoid Assay
Flavonoids were assayed using a calibration curve for quercetin, the reference molecule. A stock solution with a concentration of 100 mg/ml was prepared and then diluted to concentrations of 10, 30, 40, 50, 70 and 100 mg/ml. Subsequently, 1 ml of each solution was mixed with 0.3 ml of 10% AlCl3 solution, 1 ml of distilled water, 1 ml of 2 M NaOH and 0.3 ml of NaNO2. These prepared samples were incubated for 30 minutes at room temperature. The colorimetric absorbance values obtained were estimated using a UV-visible spectrophotometer at a wavelength of 510 nm. After obtaining the calibration curve for quercetin, the flavonoid content of our extracts was expressed as mg quercetin equivalent per gram of flower extract (mg QUE/g) [15].
Determination of Tannins
Condensed tannins were determined using the vanillin acid method described by [16]. This method is based on the ability of vanillin to react with condensed tannin units in the presence of acid to produce a coloured complex. The reactivity of vanillin with tannins involves only the first polymer unit. For this assay, 400 µl of each stock solution was mixed with 3 ml of 4% vanillin solution and 1500 µl of hydrochloric acid (HCl). A blank was prepared by replacing the reagent with the water-acid mixture. The tubes were kept at 30°C for twenty minutes in a dark room. Absorbance was measured at 500nm. Condensed tannin levels were determined by a calibration or linear calibration curve plotted using precise concentrations of catechin as the reference substance. The results are calculated according to the equation: C = (c ×D/Ci) ×100; and expressed in milligrams of gallic acid equivalent per 100 milligrams of dry extract (mgEC/100mg). C = Concentration of condensed tannins in mg EAG/100 mg dry extract; c = Concentration of the sample read; D = Dilution factor; Ci = Concentration of the initial solution.
Evaluation of the Antioxidant Activity of Individual Herbal Teas of a Mixture of Oyster Mushrooms, Ginger and Lemongrass
Evaluation of the Anti-free Radical Activity of DPPH (2.2-diphenyl-1-picrylhydrazyl)
One (1) ml of DPPH solution (concentration 0.11 mg/ml) prepared in absolute methanol was added to 50 μl of extract at concentrations of 1.25, 2.5, 5, 10 and 20 mg/dl. Ascorbic acid prepared in the same concentrations as the extracts was used as the standard. Absorbance was measured at 517 nm after incubating the solutions for 30 minutes in the dark. The control was the DPPH solution without the antioxidant. The percentage of trapping was calculated according to the following equation:
% entrapment= [(A1 - A2) / A1] x 100. Where: A1: absorbance of the control (DPPH solution without extract). A2: absorbance in the presence of extract [16].
Iron Reducing Power (FRAP)
The reducing power of the herbal teas was determined using the method of [16]. To each test tube containing 0.1 ml of sample solution (herbal tea or vitamin C) prepared at concentrations of 1.25, 2.5, 10, 20 mg/ml, were added 2 ml of distilled water followed by 2 ml of potassium hexacyanoferrate [K3Fe (CN)6] (10g/l). The mixture was incubated in a water bath at 50°C for 30 minutes. A volume of 2 ml of trichloroacetic acid (100 g/l) was then added and the mixture was centrifuged at 3000 rpm for 10 minutes. After centrifugation, 2ml of the supernatant was collected and mixed with 2ml distilled water and 0.4ml ferric chloride [FeCl3] (1g/l). The reading was measured at 700 nm.
Evaluation of the Inhibition of Digestive Enzymes by Individual Teas of a Mixture of Oyster Mushrooms, Ginger and Lemongrass
Pancreatic Alpha Amylase
The protocol used is that of [17], with some minor modifications. In test tubes, 100 µl aliquots of extract and acarbose prepared at concentrations of 1.25, 2.5, 5, 10 and 20mg/dl were added to the reaction medium; the reaction medium being a mixture of 20 µl enzyme (30 µg/ml) and 1380 µl tris-HCl buffer pH 6.8. The mixture was then preincubated at 30o C for 20min. The reaction was then initiated by adding 100 µl of starch (1%) solution. After 20 min of incubation, the enzymatic reaction was stopped by adding 2 ml of acidified iodine, and the absorbance was read at 500nm. The percentage inhibition was calculated using the following formula:
% inhibition of α-amylase = (AControl - ASample) / AControl x100.
Alpha Glucosidase
The protocol followed was that of [42] but with some minor modifications; the reaction medium consisted of: 20 μL of extract solution (sample) or acarbose solution (positive control) or phosphate buffer solution (control: total enzyme activity without inhibitor); 10 μL of enzyme solution in all tubes; 20 μL of substrate solution (1 mM pNPG) in all tubes except the blank where substrate solution is replaced by phosphate buffer; 50 μL of phosphate buffer solution (50 mM, pH 6.8) is added to all tubes. The tubes were incubated at 37°C for 40 min, after which the reaction was stopped by adding 50 μL of Na2CO3 (0.1 M). Finally, the absorbance of the para-nitrophenol released was measured using a spectrophotometer at 405 nm. The percentage inhibition was calculated using the following formula:
% inhibition of α-glucosidase = (AControl - ASample) / AControl x100.
Pancreatic lipase
The method mentioned in [18] study was slightly modified. In a 280 µL reaction system, 180 µL of pH 7.2 phosphate buffer, 40 µL of sample and 20 µL of 10 mM p-NPP (p-nitrophenyl palmitate) were added in succession and incubated at 37 ◦C for 10 min, and then 40 µL of 10 mg/mL pancrelipase solution was added, fully mixed and incubated at 37 ◦C for 15 min, followed by the immediate addition of 200 µL of absolute alcohol to terminate the reaction. The mixture was centrifuged at 10,000× g for 2 min, and the supernatant was taken. The absorbance was measured at a wavelength of 405 nm by a spectrophotometer and recorded as B1. The sample was replaced with phosphate buffer as the sample blank control and recorded as B0. The sample without inhibitors was taken to measure inhibitor-free activity, denoted A1; the corresponding negative control without enzyme was used as a negative control without inhibitor, denoted A0. Orlistat was used as a positive control.
Lipase activity inhibition (%) = (1 - (B1 - B0)/(A1 - A0)) × 100
Evaluation of Anti-inflammatory Activity Using the Egg Albumin Denaturation Method
A 5 mL solution consisting of 0.2 mL egg albumin, 2.8 mL phosphate buffered saline (PBS, pH 6.4) and 2 mL of tea at concentrations of 0.125; 0.25; 0.5; 1; 2 mg/dL was prepared beforehand. Diclofenac was used as the reference drug at the same concentrations as the teas. A double volume of distilled water was used as a control. The mixtures were incubated at 37±2°C for 15 min and then heated to 70°C for 5 min. After cooling, absorbance’s were measured at 660nm [26]. The % inhibition of protein denaturation was calculated using the formula:
% inhibition = 100 × [Vt/Vc-1] Vt = absorbance of test sample; Vc = absorbance of control [19].
Formulation of Teas Based on Mixtures of Oyster Mushrooms and Lemongrass and Oyster Mushrooms and Ginger
After analysis of the results and to boost the activities of the mushrooms, formulations based on mushrooms and ginger and those based on mushrooms and lemongrass were produced using random mixing. These are recorded in Tables 1 and 2 respectively. The proportion level for mushrooms was 70-80%, while for ginger rhizomes and lemongrass leaves was between 20-30%. Subsequently, 3 g of the various blends were packed in teabags and percolated in hot water for 15 minutes for subsequent analysis.
Evaluation of Some in Vitro Anti-obesity Physiological Aspects of Mushroom and Ginger-based Teas and Mushroom and Lemongrass based Teas and Selection of the best Teas
The tests previously carried out on these different teas taken individually were re-evaluated on the teas from the formulations. These tests were quantitative phytochemical screening (polyphenols, flavonoids and tannins), antioxidant tests (DPPH and FRAP), digestive enzyme inhibition tests (pancreatic alpha amylase, alpha glucosidase and pancreatic lipase) and the anti-inflammatory test by inhibiting the denaturation of egg albumin.
Selection of the Best Teas
The selection of the best tea for each of the two groups of teas from the blends (oyster mushroom teas supplemented with ginger and oyster mushroom teas supplemented with lemongrass) was made on the basis that it presented the best activity.
Statistical Analysis
Analyses were performed using SPSS 20.0 software. Tests were performed in triplicate. Results were expressed as mean ± standard deviation. Duncan's test was used for comparison of means. The significance level was expressed at 5%.
Results
Evaluation of Some Anti-Obesity Physiological Aspects of Mushroom Tea, Lemongrass Tea and Ginger Tea
Phytochemical Screening
Table 3 shows the phytochemical screening of the individual mushroom, lemongrass and ginger teas. The table shows that the total polyphenol, flavonoid and tannin content of the mushroom tea was significantly lower than that of the lemongrass and ginger teas.
Evaluation of Antioxidant Activity
The antioxidant activity of the various herbal teas was assessed in vitro by DPPH and FRAP tests.
DPPH Test
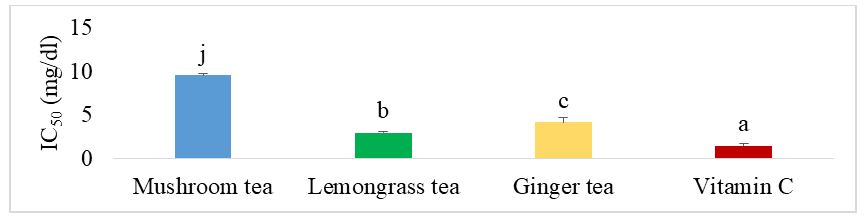
The results were expressed in terms of IC50, which is the minimum concentration capable of reducing 50% of free radicals. The lower IC50, the more effective the herbal tea. Figure 1 shows the IC50 of the different herbal teas for the DPPH test. It can be seen that the mushroom herbal tea had the highest IC50 (p˂0.05), higher than the IC50 for vitamin C (standard) and the individual lemongrass and ginger herbal teas. Vitamin C showed the lowest IC50 and therefore the highest activity (p˂0.05).
FRAP Test
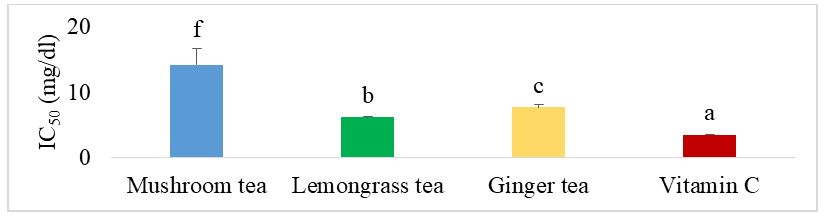
Figure 2 shows the IC50 of the different herbal teas for the FRAP test. This shows that there is a significant difference (p˂0.05) between the different herbal teas and even with vitamin C. Mushroom tea showed the lowest activity. Vitamin C showed the best reducing power with lowest IC50.
Digestive Enzyme Inhibition Test
Pancreatic Alpha-Amylase
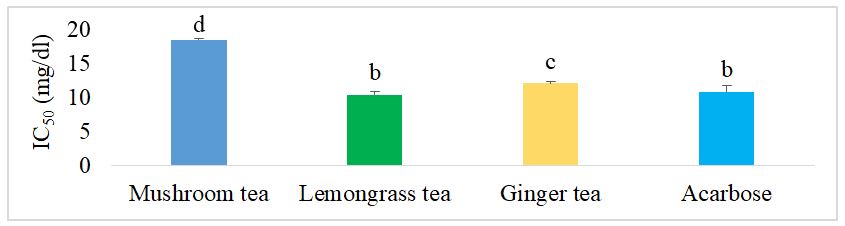
Figure 3 shows the IC50 of the different herbal teas for the alpha amylase inhibition test. It can be seen that mushroom tea had a significantly higher IC50 (p˂0.05) than acarbose, the reference drug, and the other herbal teas, ginger tea and lemongrass tea. There was also no significant difference between lemongrass tea and acarbose.
Alpha-glucosidase
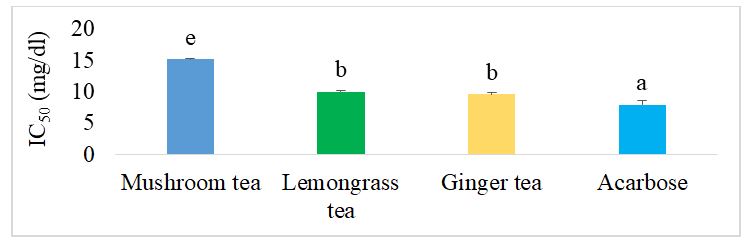
The effect of herbal teas on alpha glucosidase inhibition is shown in Figure 4 below. It can be seen that the mushroom tea showed the lowest inhibitory activity with an IC50 equal to 15.23 mg/dl. Furthermore, there was no significant difference (p˂0.05) between ginger tea and lemongrass tea. However, both showed lower activity than acarbose, the reference drug.
Lipase
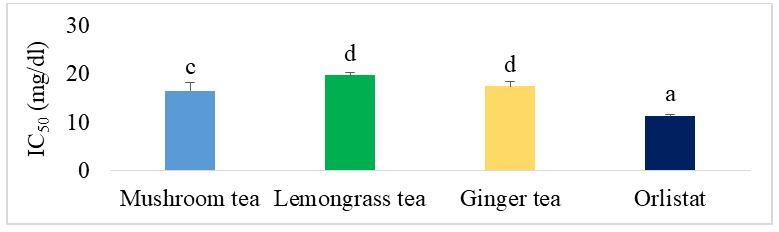
Figure 5 shows the IC50 of the different herbal teas for the lipase inhibition test. It can be seen that the mushroom tea has a significantly lower IC50 (p˂0.05) than the ginger and lemongrass teas. The mushroom tea also showed significantly less activity (p˂0.05) than orlistat, the reference drug.
Anti-inflammatory Activity Using the Egg Albumin Denaturation Method
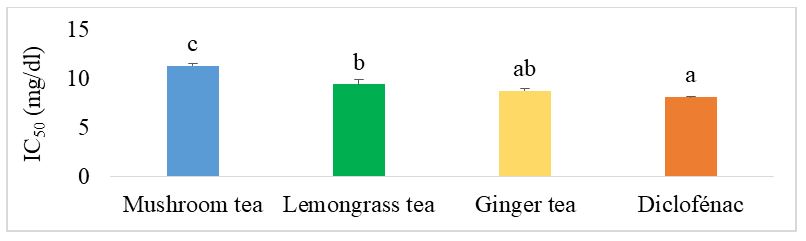
Figure 6 shows the IC50 values of the different herbal teas compared with diclofenac for inhibition of egg albumin denaturation. It can be seen that mushroom herbal tea showed the lowest anti-inflammatory activity with the highest IC50 at 11.390 mg/dl. Also, no significant difference (p˂0.05) was observed between ginger and diclofenac, the reference drug.
Formulation of Teas
Ten formulations were obtained, including five made from mushrooms and ginger, ranging from G1 to G5, and five others made from mushrooms and lemongrass, ranging from C1 to C5.
Evaluation of Some Anti-Obesity Physiological Aspects of the Herbal Teas Formulated
Phytochemical Screening of Different Formulations
Tables 4 and 5 below show the phytochemical screening of mushroom-based teas supplemented with ginger and mushroom-based teas supplemented with lemongrass respectively. For the mushroom- and ginger-based teas, G1 and G2 had the highest polyphenol content, G4 the highest flavonoid content and G3 and G5 the highest tannin content.
For mushroom-based teas supplemented with lemongrass, C3 and C5 had the highest polyphenol content and C1 the highest flavonoid and tannin content. All of these 10 herbal teas had significantly higher levels of these metabolites (p˂0.05) than the mushroom teas alone.
Antioxidant Activity of the Different Formulations
DPPH Test
Figure 7 shows the inhibitory concentration 50 (IC50): the concentration at which the antioxidants present in the extracts inhibit 50% of the free radicals. It can be seen that the best oyster mushroom and ginger-based herbal teas were G1, G2 and G5, while the best oyster mushroom and lemongrass-based herbal tea was C1. It should be noted that all these different formulated herbal teas had IC50 values significantly lower (p˂0.05) than that of the mixed mushroom herbal tea alone but significantly higher (p˂0.05) than that of vitamin C, the reference drug. The best oyster mushroom tea supplemented with lemongrass C1 showed greater activity (p˂0.05) than the best oyster mushroom teas supplemented with ginger G1, G2 and G5.
FRAP Test
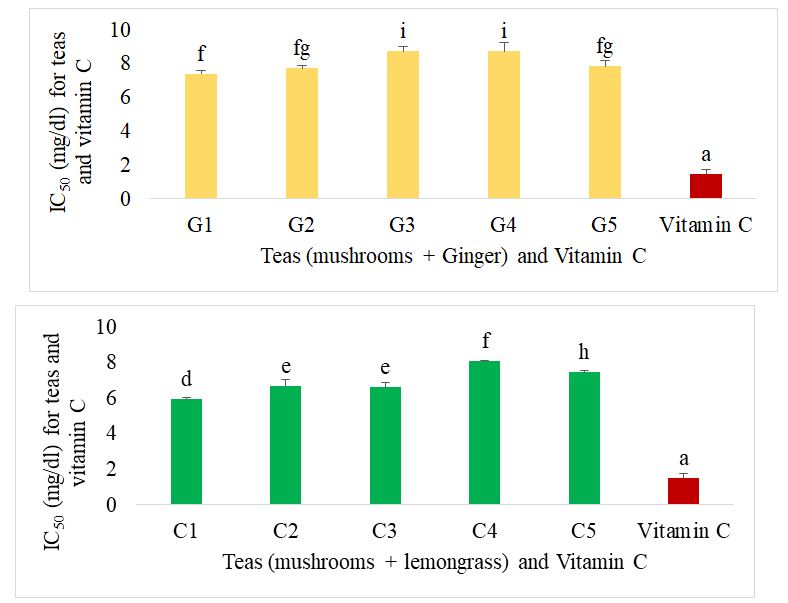
The IC50 of the different herbal teas formulated against vitamin C for the FRAP test is shown in Figure 8. It can be seen that for the oyster mushroom and ginger teas, the best teas were G1 and G4, while C1 and C4 were the best oyster mushroom and lemongrass teas. There was no significant difference between these best teas. All the teas showed a high antioxidant power compared with mushroom tea alone. Vitamin C had the lowest IC50 and therefore a significantly higher reducing power than all the formulations.
Digestive Enzyme Inhibitory Activity of the Different Formulations
The results were expressed as IC50 values.
Alpha Amylase Inhibition Test
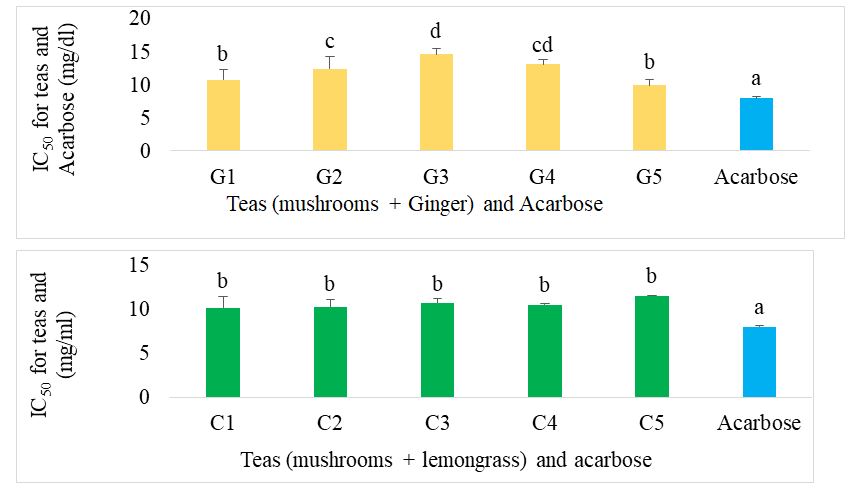
Figure 9 shows the IC50 of the various teas formulated compared with acarbose for alpha-amylase inhibition. Among the ginger-supplemented mushroom-based teas, G1 showed the best activity, similar to that of acarbose. In the lemongrass-supplemented mushroom-based teas, C1, C2 and C3 showed the best activity, significantly higher (p˂0.05) than acarbose, G1 and the individual teas.
Alpha-glucosidase Inhibition Test
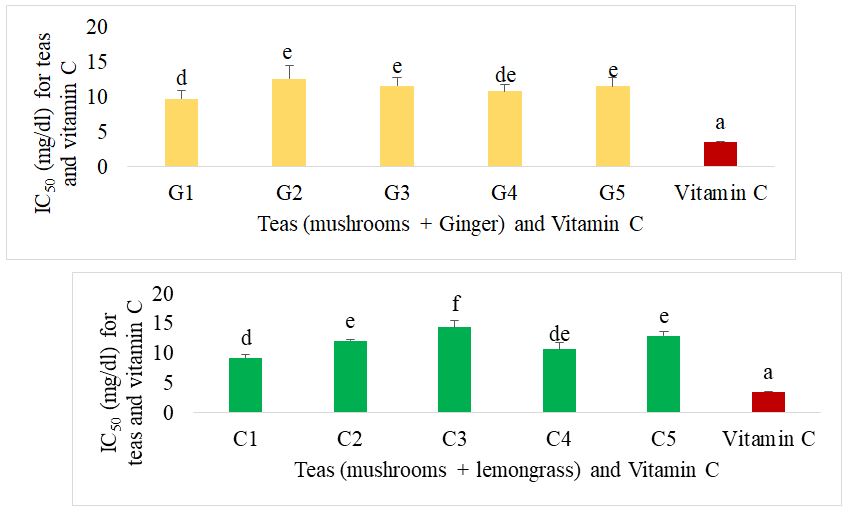
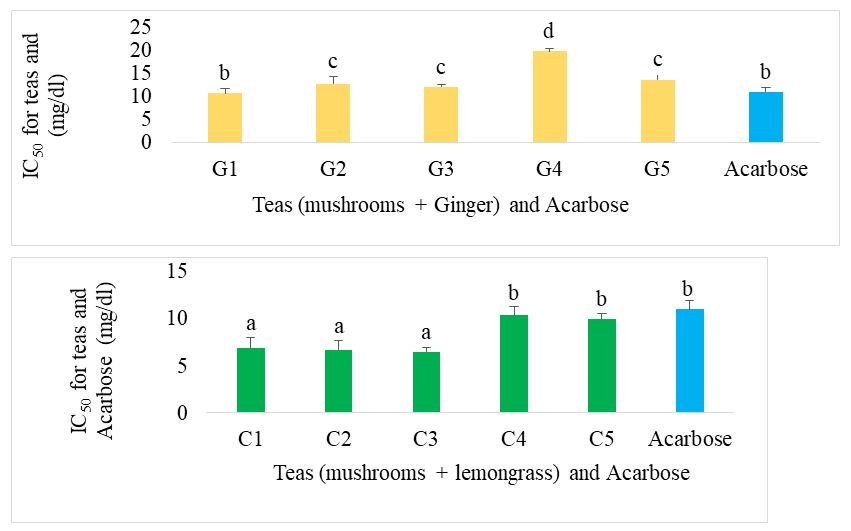
The IC50 of the different formulated teas compared with acarbose for alpha glucosidase inhibition are presented in figure 10. For mushroom-based teas supplemented with ginger, the best teas were G1 and G5. There was no significant difference (p˂0.05) between the mushroom and lemongrass teas. These showed an IC50 significantly equal to those of G1 and G5 teas. Acarbose showed the best IC50 compared with all the different herbal teas.
Lipase Inhibition Test
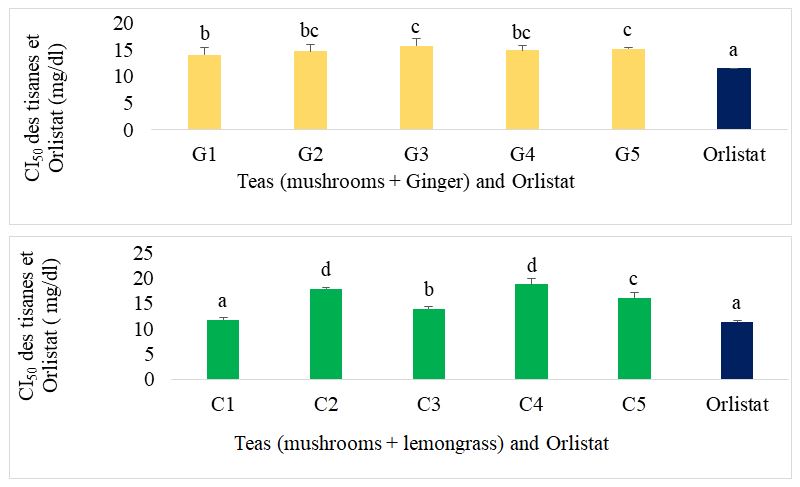
Figure 11 shows the IC50 of the different formulated teas compared with orlistat for lipase inhibition. In the case of ginger-supplemented mushroom-based teas, the best teas were G1, G2 and G4. Their IC50 was significantly lower than that of the individual teas (mushroom tea, lemongrass tea and ginger tea) and significantly higher than that of orlistat, the reference drug. Mushroom tea supplemented with lemongrass C1 showed the best activity and was similar to orlistat.
Anti-Inflammatory Activity by Inhibiting the Denaturation of Egg Albumin by the Different Formulations
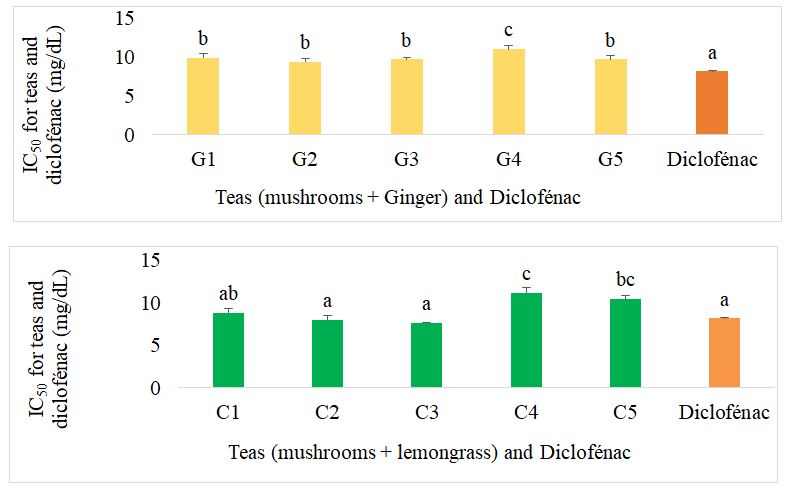
The IC50 of the different formulated teas compared with diclofenac for inhibition of albumin denaturation are presented in figure 12. Among the mushroom- and ginger-based teas, with the exception of G4, there was no difference between the different teas. Teas G1, G2, G3 and G5 showed the best activity, but this was lower than the activity of diclofenac, the reference drug. For mushroom-based teas supplemented with lemongrass, C1, C2 and C3 showed the best activity, similar to that of diclofenac. These teas had lower IC50 values than ginger mushroom tea, lemongrass tea and mushroom tea.
Selection of the Best Teas
The results of these tests show that the G1 mushroom and ginger tea (2.1g oyster mushrooms and 0.9g ginger) and the C1 mushroom and lemongrass tea (2.1g oyster mushrooms and 0.9g lemongrass) rank among the top three teas, with the best activity in most tests. Thus, they were identified as the best formulations. Tea C1 demonstrated greater activity than G1.
Discussion
Herbal teas have long been used in traditional medicine to treat many diseases and abnormal physiological conditions, such as obesity. According to modern medicine, the main cause of obesity is excess energy intake relative to expenditure, accompanied by hyperlipidaemia, hyperglycaemia, insulin resistance, lipid peroxidation, chronic inflammation and other symptoms [1,5]. It is difficult to achieve a global effect with a single targeted therapy in the context of obesity and other chronic diseases with multiple metabolic disorders [20]. Hence, the aim of this study was to formulate mushroom teas supplemented with ginger or lemongrass and to evaluate some anti-obesity aspects in vitro through phytochemical screening, assessment of antioxidant, digestive enzyme inhibitory (alpha amylase, alpha glucosidase and pancreatic lipase) and anti-inflammatory activities. Initially, tests carried out on individual mushroom, lemongrass and ginger teas showed that the mushroom teas had lower levels of phenolic compounds, antioxidant activity, inhibition of digestive enzymes (alpha amylase and alpha glucosidase) and anti-inflammatory activity than the lemongrass and ginger teas and even the reference medicines, with the exception of the lipase inhibition test. The difference in phenolic compounds between these three teas could be explained by their different genetic make-up, climatic factors, stage of development and harvesting period... Also, the phenolic compound contents of ginger and lemongrass were lower than those obtained respectively by [8,21]. This difference could be explained by the nature of the soils, variations in climate, the harvesting period and the growing season [22-24]. In fact, the low activity of mushroom teas compared to the other two herbal teas could be explained by the high levels of phenolic compounds in ginger and lemongrass herbal teas, as presented in the phytochemical screening. Furthermore, the ability of mushroom teas to inhibit lipase could be attributed to their composition in saponins, which can reduce the interaction of lipase with the substrate by aggregating with fat droplets to form micelles [25]. Mushrooms are also rich in chitin, which has excellent emulsification properties, inhibiting pancreatic lipase [26]. In addition, ten formulations were obtained following random blending, which were intended to boost the activities of the mushroom herbal teas. Among these ten formulations, five were based on mushrooms and ginger rhizomes (G1- G5) and another five on mushrooms and lemongrass leaves (C1-C5).
Antioxidant activity was also assessed using DPPH and FRAP tests. The results showed that all 10 herbal teas were more active than mushroom tea alone. These results corroborate those of [27] who showed that supplementation with 2.5 % Zingiber officinale increased the antioxidant capacity of Aloysia citadora herbal tea. The ability to inhibit free radicals and the reducing power of the teas formulated could be explained by the presence of polyphenols, in particular flavonoids, which have the ability to donate hydrogen atoms or electrons mainly from their A ring hydroxyls [28]. In addition, there was a significant positive correlation (P ˂ 0.01) between polyphenol, flavonoid and tannin content and antioxidant activity. These results are similar to those of [29] who showed a positive and significant correlation between antioxidant activity and polyphenol content. The higher antioxidant activity of the formulated teas compared with mushroom tea alone could be explained by the increased quantity and quality of polyphenols, but also by the presence of compounds such as gingerol from ginger, citral from lemongrass and many others, which are known to be powerful antioxidants [30,31]. Nevertheless, the difference in results between the different teas formulated could be explained by the number of free -OH groups.
With regard to inhibition of alpha-amylase activity, the best mushroom- and ginger based tea, G1, showed similar activity to acarbose, while the mushroom- and lemongrass-based teas, C1, C2 and C3, showed higher activity than acarbose. For the inhibition of alpha glucosidase, all the mushroom- and lemongrass-based teas (C1-C5) showed the same activity as the G1 and G5 teas, but this was significantly lower than that of acarbose. Pancreatic alpha amylase is a hydrolase which catalyses the breakdown of starch by acting on the α-1,4 bonds, releasing alpha dextrins and disaccharides. Alpha glucosidase acts by hydrolysing disaccharides via α-1,4 bonds into glucose molecules. These two enzymes play an important role in glycaemic control [13]. Inhibition of these two enzymes would inhibit the digestion and absorption of blood glucose and could be effective in reducing postprandial hyperglycaemia. Reducing postprandial hyperglycaemia prevents the absorption of glucose into adipose tissue, inhibiting the synthesis and accumulation of triacylglycerol.
The antihyperglycaemic capacity of herbal teas could be explained by the fact that the phenolic compounds present in mushrooms, ginger and lemongrass, in particular flavonoids, saponins and tannins, like acarbose, could bind competitively to the binding site of these enzymes, thus preventing the formation of the hydrogen bond between the amino acids at the enzyme binding site and the polar (-OH) groups in the carbon chain of the substrate [32]. On the other hand, lemongrass contains luteolin, a powerful antihyperglycaemic agent that could bind non-competitively to alpha glucosidase via hydrogen bonds and Van der Walls bonds, thus rearranging the structure of the enzyme [33]. In addition, there is a significant positive correlation (P˂0.01) between flavonoid content and alpha glucosidase inhibition. Studies have also shown that beta glucans contained in mushrooms inhibit the activity of alpha amylase and alpha glucosidase in vitro [34]. In addition, it should be noted that all the best herbal teas formulated showed superior activity to individual mushroom teas and in some cases to individual ginger and lemongrass teas. The results obtained from the formulated teas are also superior to those of [10], who evaluated the alpha amylase inhibitory activity of aqueous extracts from a mixture of mushrooms. The difference in results could be explained by the supplementation of the teas with lemongrass or ginger and by the extraction method, which was maceration in their work and infusion in this study.
In terms of inhibition of pancreatic lipase activity, mushroom and lemongrass C1 herbal tea showed an effect comparable to that of orlistat and superior to the best mushroom and ginger herbal teas, as well as to the three individual herbal teas. Pancreatic lipase is a glycoprotein that hydrolyses the ester bonds of triglycerides in positions 1 and 3, releasing monoacylglycerols, glycerol and fatty acids. These absorbed fatty acids are then stored in adipose tissue. Inhibition of pancreatic lipase therefore prevents the digestion of lipids, reducing postprandial absorption of fatty acids and their storage in the body's adipocytes, leading to a reduction in body fat and hence weight loss [35]. The ability of the formulated teas to inhibit pancreatic lipase activity could be explained by the fact that the polyphenols they contain may act like orlistat by binding competitively to the enzyme's active site. In addition, there was a significant positive correlation (P˂0.05) between inhibition of lipase activity and polyphenol content. Our results are superior to those obtained by [36] who evaluated the in vitro anti-lipase activity of tea made in Georgia. The difference in results could be explained by the phytochemical composition of the different plants.
Anti-inflammatory activity was assessed by denaturing egg albumin. Studies have shown that albumin, as well as being an antioxidant, has anti-inflammatory properties in vitro and in vivo. It inhibits the production of IFN-gamma and TNF-alpha, major pro-inflammatory mediators involved in the inflammation of adipocytes in obesity [37-39]. Furthermore, in obesity, albumin levels in the body decrease. Furthermore, hypoalbuminemia is thought to reflect the degree of adiposity, inflammation and even lipotoxicity [40]. Thus, inhibition of albumin denaturation could inhibit inflammation in obese subjects. The ability of formulated herbal teas to prevent albumin denaturation could be explained by the presence of phenolic compounds such as quercetin, gallic acid and many others that could stabilise the structure of albumin through hydrogen bonds and hydrophobic interactions [41]. In addition, there is a significant and positive correlation (P˂0.05) between polyphenol content and anti-inflammatory activity.
Conclusion
The ultimate aim was to formulate teas based on a mixture of oyster mushrooms supplemented with ginger or lemongrass and to assess their effects on a number of anti-obesity physiological aspects in vitro. The results showed that ten formulations were obtained, including five consisting of oyster mushrooms and ginger (G1-G5) and five others of oyster mushrooms and lemongrass (C1-C5). The best formulations for each group were G1 (2.1g of oyster mushrooms + 0.9g of ginger) and C1 (2.1g of oyster mushrooms + 0.9g of lemongrass), which showed the best IC50 and therefore the best activities for antioxidant tests (DPPH and FRAP), inhibition of digestive enzymes (alpha amylase, alpha glucosidase, lipase) and anti-inflammatory tests, superior to the mixture of simple oyster mushrooms. The results demonstrated that supplementation with ginger and lemongrass boosted the efficacy of our mushroom teas. Nevertheless, C1 tea showed the best activity in most cases compared with G1.
Conflict of Interest
There is no conflict of interest between authors of this article.
- Yan XT, Zhang W, Zhang Y, Zhang Z, Chen D, et al. (2022) In Vitro Anti-Obesity Effect of Shenheling Extract (SHLE) Fermented with Lactobacillus fermentum grx08. Foods, 11: 1221.
- World Health Organization (2019) Rapport de suivi des 100 indicateurs clés de Santé du Cameroun en 2019. Yaoundé. (2019). 138p. http://onsp.minsante.com.
- World Health Organization (2016) Profil sanitaire analytique du Cameroun. (2016). 145 p. Site web : http:/ww w.afro.who.int/fr/Cameroun.
- Faucher P. et Poitou C (2015) Physiopathologie de l’obésité. Revue du rhumatisme monographies - Elsevier Masson. 7.
- Junj UJ, Choi MS (2014) Obésité et ses complications métaboliques : le rôle des adipokines et la relation entre obésité, inflammation, résistance à l’insuline, dyslipidémie, stéatose hépatique non alcoolique. Int. J. Mol. Sci. 15: 6184-223
- Maftoun P, Johari H, Soltani M, Malik R, Othman N, El Enshasy H, (2015) The edible mushroom Pleurotus spp. : I. Biodiversity and nutritional values. International Journal of Biotechnology for Wellness Industries, 4: 67-83
- Oladeji OS, Adelowo FE, Ayodele DT. et Odelade KA (2019) Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Scientific African, 6.
- Fotsing S, Ngogang MP, Nganchouko B, Simo LJ, Ngogang J (2022) Teneurs en polyphenols, flavonoids et activités antioxydantes des rhizomes de Zingiber officinale produits dans cinq sites de culture au Cameroun. Revue de l’académie des sciences du cameroun. 18: 397-406.
- Etoundi O, Mbang A, Tuem R, Gouado I (2017) Study of Toxicity and Antidiabetic Activity of Ethanolic and Hydroethanolic Extracts of Pleurotus pulmonarius and the Aqueous Extract of Pleurotus floridanus. J Food Nutr Popul Health, 1: 20.
- Mbang A, Etoundi O, Djessissem R, Kana M (2020) Evaluation of the activity of alpha amylase and antioxidant potential formulations of three varieties of oyster mushrooms: pulmonarius, floridanus and sajor-caju. Journal of the cameroon academy of sciences, 15: 1-12.
- Chieguen T, Dongmo A, Mbang M et al. (2023) Antihypertensive, ameliorating effect on lipid profile and oxidative stress markers of aqueous extract of Pleurotus floridanus in rats. American journal of medical and clinical sciences, 8: 1-7.
- Nkepndep S, Dongho D, Lienou L et al. (2022) Antioxidant and antiobesogenic properties of aqueous extracts of Hibiscus sabdariffa, Zingiber officinale and Mentha spicata in wistar high-fat diet rats. Journal of Food and Nutrition Sciences, 10: 151-64
- Vasi´c D, Katani´c Stankovi´c JS, Uroševi´c T, Kozarski M, Naumovski N, Khan H, et al. (2024) Insight into Bioactive Compounds, Antioxidant and Anti-Diabetic Properties of Rosehip (Rosa canina L.)-Based Tisanes with Addition of Hibiscus Flowers (Hibiscus sabdariffa L.) and Saffron (Crocus sativus L.). Beverages, 10: 1.
- Etoundi O, Kayo V, Mbang A, Piéme C (2019) Study of acute toxicity and the effect of the aqueous extract of a formulation of three edibles mushrooms on oxidative stress induced in rats. World Journal of Food Science and Technology. 3: 6-13.
- Nidal Jaradat, Mohammad Qneibi, Mohammed Hawash, Anood Sawalha, Sana Qtaishat, et al. (2020) Chemical Composition, Antioxidant, Antiobesity, and Antidiabetic Efects of Helichrysum sanguineum (L.) Kostel. from Palestine. Arabian Journal for Science and Engineering.
- Mignanwandé (2020) Ethnomedicinal, phytochemistry and antioxidant activity studies of Crateva adansonii DC (Capparidaceae) in the commune of Cotonou and Dassa-Zoumè in Benin.
- Thalapaneni NR, Chidambaram KA, Ellappan T, Sabapathi ML, Mandal SC (2008) Inhibition of carbohydrate digestive enzymes by Talinum portulacifolium (Forssk) leaf extract. Journal of Complementary and Integrative Medicine, 5: 213-20.
- Kim YS, Lee YM, Kim H, Kim J, Jang DS, et al. (2010) Anti-obesity effect of Morus bombycis root extract: Anti-lipase activity and lipolytic effect. J. Ethnopharmacol, 130: 621-4.
- Fetni S. et Bertella N (2020) Etude in vitro des propriétés anti-inflammatoires de l’extrait méthanolique des fruits de Rosa canina L.(Rosacées). Nutr.santé, 9: 117-25.
- Rodgers RJ, Tschop MH, Wilding JP (2012) Médicaments anti-obésité : passé, présent et futur. Dis. Model. Mech, 5: 621-6
- Basera P, Lavania M, Agnihotri A, Lab B (2019) Analytical investigation of Cymbopogon citratus and exploiting the potential of developed silver nanoparticule against the dominatic species of pathogenic bacteria. Front microbiol, 10: 282
- Malik F, S Hussain, A Sadiq, G Parveen, A Wajid, S Shafat, RA Channa, et al. (2012) Phyto-chemical analysis, anti-allergic and anti inflammatory activity of Mentha arvensis in animals. African Journal of Pharmacy and Pharmacology, 6: 613-9.
- Sujana P, Sridhar TM, Josthna P, Naidu CV (2013) Antibacterial activity and phytochemical analysis of Mentha piperita L. (Peppermint) - an important multipurpose medicinal. plantAm. J. Plant Sci., 4: 77-83.
- Akhtar N, Ihsan-ul-Haq, Bushra Mirza (2015) Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arabian Journal of Chemistry, 11: 1223-35.
- Karu N, Reifen R. et Krem Z (2007) Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. Journal of agricultural and food chemistry, 55: 2824-8.
- Barkhordari MR, Fathi M (2018) Production andcharacterization of chitin nanocrystals from prawn shell and their application for stabilization if pickering emulsions. Foods hydrocolloids, 82: 338-45.
- Yeo M, Soro L, Koffi E, Coulibaly L (2021) Impact of ginger enrichment on biochemical characteristics of tisane from Aloysia Citadora leaves, cultivated a small scale in the area of man (West region of Côte d’Ivoire). International journal of Environment and agriculture research, 7: 66-72.
- Kintzios S, Papageorgiou K, Yiakoumettis I, Baricevic D, Kusar A (2010) Evaluation of the antioxidants activities of four Slovene medicinal plant species by traditional and novel biosensory assays. Journal of Pharmaceutical and Biomedical Analysis, 53: 773-6.
- Bamba B, Benie CKD, Ouattara A, Doukourou DN, Kamou RK et Ouattara K (2021) Teneurs en polyphenols totaux, activités antioxydantes des macérés et décoté des feuilles de Uvaria chamae P. Beauv. (Annonaceae). International journal of biological and chemical sciences, 15: 54-67.
- Bouzenna H, Hfaiedh N, Giroux-Metges MA, Talarmin H (2017) Biological properties of citral and its potential protective effects against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed pharmacother, 87: 653-60
- Preciado-Ortiz ME, Martinez-Lopez E, Pedraza-Chaverri J, Medina-Campos ON, Rodriguez-Echevarria R, Reyes-Pérez SD, Rivera-Valdès JJ (2024) Gingerol increases antioxidant enzymes and attenuate lipopolysaccharide induced inflammation by modulating adipokines in 3T3-L1 adipocytes. Antioxidants, 13: 1093
- Faiveley M (2010) Biochemical and chemical processes in agri-food. Vegetable products. Making beers. Techniques-engineer. ENILBIO poligny. France.
- Djeudjo E, Ragazzi E, Urettini M, Sauro B, Cichero E, Tonelli M, Droldi G (2022) Magnolol and lutrolin inhibition of α-glucosidase activity : kinetics and type of interaction detected by in vitro and in silico studies. Pharmaceuticals, 15: 205.
- Wunjuntuk K, Ahmad M, Techakriengrai T, Chunchom R (2022) Proximate compoqition dietary bibre, beta glucan content, and inhibition of key enzymes linked to diabetes and obesity in cultivated and wild mushrooms. Journal of composition and analysis, 10: 104226.
- Mizutani T, Inatomi S, Inazu A, Kawahara E (2010) Hypolipidemic effect of Pleurotus eryngii extract in fat-loaded mice. J Nutr Sci Vitaminol, 56: 48-53.
- Gulua L, Nikolaishvili N, Jgenti M, Turmanidze T, Dzneladze G (2018) Polyphenol content, anti-lipase and antioxidant activities of tea mades in Georgie. Annals of agrarican science, 16: 357-61
- Lang JD, Figueroa M, Chumley P, Aslan M, Hurt J, Tarpey MM, Alvarez B, Radi R, Freeman BA (2004) Albumin and hydroxyethyl starch modulate oxidative inflammatory injury to vascular endothelium. In : Anesthesiology. 100: 51-8.
- Powers KA, Kapus A, Khadaroo RG, He R, Marshall JC, Lindsay TF, Rotstein OD (2003) Twenty-five percent albumin prevents lung injury following shock/resuscitation. In: Crit Care Med. 31: 2355-63.
- Bar-Or D, Thomas GW, Bar-Or R, Rael LT, Scarborough K, Rao N. Shimonkevitz R (2006) Commercial human albumin preparations for clinical use are immunosuppressive in vitro. In Crit Care Med, 34: 1707-12.
- Farigon N, Montel F, Mlolanne M. et Boirie Y (2014) Hypoalbuminémie du sujet obèse non agressé : marqueur de dénutrition ou de surnutrition ? Nutrition clinique et Métabolisme, 28: 49-50.
- Podlipnik, Nataaia, Poklar, (2012) Interactions of different polyphenols with bovine serum albumin usingfluorescence quenching and moleculardocking. Food chemistry, 135: 2418-24.
- Deguchi Y, Miyazaki K (2010) Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutrition & metabolism, 7: 1.

Tables at a glance
Figures at a glance