Efficacy Analysis of Autologous Blood-Assisted Temporal Internal Limiting Membrane Flap Technique in Treating Macular Holes
Received Date: June 23, 2025 Accepted Date: July 07, 2025 Published Date: July 10, 2025
doi: 10.17303/jooa.2025.9.104
Citation: Weifang Ma, Li Zhou, Yali Liu, Yuchuan Chen (2025) Efficacy Analysis of Autologous Blood-Assisted Temporal Internal Limiting Membrane Flap Technique in Treating Macular Holes J Ophthalmol Open Access 9: 1-12
Abstract
Purpose: Exploring the therapeutic effect of autologous blood combined with temporal inverted internal limiting membrane (ILM) flap technique.
Methods: This retrospective study includes patients with macular holes (MH) with a minimum diameter exceeding 400 µm or high myopia MH. The autologous blood combined with the temporal inverted ILM flap technique (temporal group) was applied to 14 eyes, and the autologous blood combined with the inverted ILM flap technique (classic group) was applied to 15 eyes.
Results: Both groups demonstrated improvement in BCVA. There was no difference in EZ/ELM repair rate between the two groups (P = 0.05). However, the incidence of complications was lower and U/V closure rate was higher in the temporal group (P = 0.001, P = 0.03). Additionally, one case of "flap closed" persisted for more than three months. In the temporal group, the thickness of the retina outside the macular fovea by 1.5–3 mm did not significantly decrease, while a decrease was observed in the classic group. The average follow-up duration for the two groups was 2.86 ± 1.4 months and 3.86 ± 1.6 months, respectively (P = 0.09).
Conclusion: The method of combining autologous blood with an inverted ILM flap technique for treating large diameter MH or HM-associated MH demonstrates a lower incidence of complications compared to the classical inverted ILM flap technique and can achieve good visual and anatomical recovery after surgery. "Flap closed" may represent the final state of MH closure. Furthermore, the temporal inverted ILM flap technique appears to have a relatively small impact on retinal thickness.
Keywords: ILM Peeling; Macular Hole; Pars Plana Vitrectomy; Temporal Inverted ILM Flap; Inverted ILM Flap
Abbreviations
Limiting Membrane - ILM; Macular Holes - MHs; Best Corrected Visual Acuity - BCVA; Ellipsoid Zone/External Limiting Membrane - EZ/ELM; Full-Thickness MHs - FTMHs; Highly Myopic - HM; Perfluorocarbon Liquid - PFCL; Disc Diameter - DD; Optical Coherence Tomography - OCT; Ophthalmic Viscoelastic Devices - OVDs; Autologous Blood Clots - ABC; Logarithm of The Minimum Angle of Resolution - logMAR
Background
The standard therapy for small-sized macular holes (MHs) (< 400 µm) is vitrectomy with internal limiting membrane (ILM) peeling, achieving a closure rate exceeding 90% with good functional outcomes. However, large (> 400 µm in diameter), chronic, and persistent MHs pose challenges for surgeons, as increasing hole size is associated with lower closure rates and poorer functional outcomes [1]. According to reports, the closure rate for full-thickness MHs (FTMHs) with a diameter greater than 400 µm ranges between 50% and 75% [2-3]. The inverted ILM flap technique can prevent a flat and open appearance of the MH postoperatively and improve both functional and anatomical outcomes in MHs larger than 400 µm. Compared with standard surgery, the use of the inverted ILM flap technique results in better restoration of the foveal anatomical structure and visual improvement, with a closure rate of up to 98% [3]. Multiple studies have reported similar favorable outcomes and have further extended the application of this technique to highly myopic (HM) MHs [4-6].
However, large-area ILM peeling can lead to a reduction in retinal correspondence sensitivity thresholds [7-8]. Additionally, 360-degree ILM peeling poses risks such as flap loss. Michalewska et al. [9] attempted to improve the technique by reducing the area of ILM peeling and termed this modified surgery the "temporal inverted ILM flap technique". This technique is less complex and achieves hole closure rates and visual prognosis similar to the classic inverted flap technique. The unpeeled nasal ILM is more likely to restrict retinal displacement towards the optic disc, potentially facilitating hole closure [10].
Nevertheless, there is no unified standard for the size range of peeled ILM, and further comparison with traditional surgical techniques is necessary to assess postoperative glial proliferation and changes in retinal thickness. To reduce reoperations caused by flap displacement, some surgeons utilize adjuvants such as viscoelastic agents and perfluorocarbon liquid (PFCL) [11-16]. However, using these materials may increase surgical costs and pose additional complications, such as PFCL residue. Autologous blood is also a commonly used adjuvant. Compared to other potential blood components such as platelets, plasma, or serum, fresh blood is more visible and may be easier to handle during application [17-18]. Moreover, no additional procedures or instruments are required to process fresh blood. Currently, there are limited reports on the application of autologous blood-assisted temporal flap techniques. Therefore, this study aims to evaluate the efficacy of autologous blood combined with the temporal flap in treating MHs.
Methods
This retrospective case-control study analyzed patients with MHs treated in our department since March 2020 to November 2024.All surgical procedures are performed by an experienced surgeon under local anesthesia. The inclusion criteria were as follows:
Minimum MH diameter > 400 µm or HM with MHs. Eyes with HM macular degeneration were not excluded. The exclusion criteria included a history of glaucoma, proliferative diabetic retinopathy or diabetic macular edema, age-related macular degeneration, submacular neovascularization, and other ocular diseases that could affect the visual assessment during postoperative follow-up.
The two datasets were independent and characterized by small sample sizes. Statistical analysis included the following: Categorical variables: Chi-square tests or independent t-tests; and continuous variables: Independent t-tests or Mann–Whitney U tests based on data distribution. All analyses were performed with Statistical Package for the Social Sciences software (version 23; IBM Corporation, Armonk, NY, USA), with statistical significance defined as two-tailed (P < 0.05).
Surgical techniques include the classic inverted ILM flap technique and the temporal inverted ILM flap technique. The classic group, treated with the inverted ILM flap technique, included 14 patients (15 eyes). The temporal group, treated with the temporal inverted ILM flap technique, consisted of 13 patients (14 eyes).All patients underwent a complete ophthalmic evaluation, including best-corrected visual acuity (BCVA) using the Snellen chart, slit lamp examination, fundus examination, and optical coherence tomography(OCT).The patient is required to schedule follow-up visits 1 week, 1 month, 3 months, and 6 months after surgery, However, due to various reasons, we were unable to collect complete data for all these time points. Therefore, we uniformly used data from postoperative follow-ups beyond 3 months for analysis. The surgical method followed was basically the same as that reported by Michalewska [9]. In the classic group, the ILM peeling range was a circular radius of 2-disc diameter (DD) around the MH, and vitrectomy probes were used to trim the ILM flaps. In the temporal group, the ILM flap diameter was selected as 1.5–2 DD. The dye used for staining was 0.25% indocyanine green, and autologous blood was used as an adjuvant for ILM flap fixation. Using a retrobulbar needle, apply 2-3 drops of blood to stabilize the ILM flap. Wait 10-20 seconds for blood coagulation, then initiate the fluid-air exchange. The infusion valve does not need to be closed. In the temporal group, 5 eyes received air and 9 eyes received C3F8 as the tamponade agent. In the control group, 6 eyes received air, 6 eyes received C3F8, and 3 eyes received silicone oil as the tamponade agent. Silicone oil was removed at 2 months postoperatively.
Patients with cataracts underwent simultaneous cataract surgery. Patients who undergo combined cataract and vitreoretinal surgery typically have more advanced cataracts. Postoperative visual acuity in these cases more accurately reflects the true surgical outcomes because the cataract—a major confounding factor—has been eliminated. In contrast, patients who undergo vitreoretinal surgery alone usually have milder cataracts that do not significantly interfere with surgical visualization or postoperative visual recovery. Hence, cataract surgery is not performed concurrently. However, when cataract extraction is combined with vitreoretinal surgery, the postoperative visual outcomes provide a more reliable assessment of the retinal procedure’s efficacy, as optical media opacities are no longer a limiting factor.
One eye had an artificial lens before surgery, and no lens opacity was observed in the remaining transparent lenses during follow-up. All surgeries were performed using a 23G glass cutting system (Stellaris PC, Bausch & Lomb). The optical coherence tomography imaging was conducted using the RS-3000lITE, NIDEK. The minimal diameter was defined as the shortest distance between the edges of the MH with OCT caliper.
Ethics approval was not required for this study as it involved a retrospective analysis of deidentified clinical data obtained from routine surgical procedures without patient intervention or privacy concerns. All patients signed an informed consent.
Results
Three eyes with HM MH and retinal detachment (one case in the temporal group filled with gas and two cases in the classic group filled with silicone oil and silicone oil removed after two months) were included. The preoperative baseline data is summarized in Table 1.
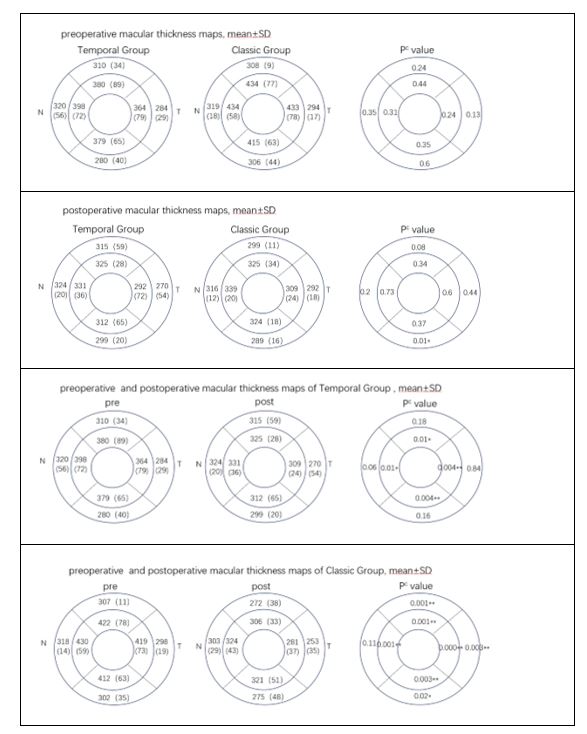
The postoperative observation indicators include BCVA, hole closure rate, macular contour, photoreceptor (ellipsoid zone/external limiting membrane (EZ/ELM)) recovery, macular thickness, and postoperative complications. The postoperative macular contour is classified based on OCT into different categories: U-shaped, V-shaped, W-shaped (irregular morphology), flat closed, flat open, and "flap closed" morphology [19]. There was a statistically significant change in BCVA in the temporal group before and after surgery (P = 0.04), whereas the classic group did not exhibit a statistically significant change in BCVA (P = 0.05). The postoperative recovery status is presented in Table 2. The changes in macular thickness before and after surgery are presented in Table 3.
Discussion
The reported diameter of the ILM temporal lobe ranges from 1/3 DD to 2 DD, [7,9,10,13-15] with no unified standard. The range of ILM flap peeling required for the classic ILM peeling technique in the past was 2 DD around the tear hole. Studies have demonstrated that a larger ILM flap area and younger age are significantly positively correlated with ILM valve contraction [20]. If the range of ILM flap removal is too small, it may be insufficient to release the retina surrounding the MH, or the flap may not cover the MH. If the removal is too large, it may lead to a decrease in corresponding visual sensitivity [7]. Therefore, in this study, the flap diameter for the temporal group was set at 1.5–2 DD.
Flap displacement can directly result in MH closure failure, necessitating reoperation and increasing medical expenses. Michalewska et al. [9] compared MH treatment using the classic inverted ILM flap technique and the temporal inverted ILM flap technique without adjuvant support. In each group, 7.0% and 6.8% (3/43 and 3/44, respectively) of patients required repeat surgery due to flap displacement. Koçak et al. [11] achieved flap stability without the need for ophthalmic viscoelastic devices (OVDs) by facilitating the PFCL flow from the temporal to the nasal side. This method achieved a perforation closure rate of 96.4% (n = 27/28) through the first surgery. Similarly, Chou et al. [15] utilized PFCL injection and OVD technology to treat 39 patients with MH without flap displacement. However, this approach requires a special type of viscoelastic agent, which may not be available in all hospitals. Additionally, using PFCL increases medical costs and may lead to complications such as PFCL residue. Blood is a commonly used adjuvant in MH surgery. Due to the barrier of ILM at the pore, red blood cells do not enter the subretinal space, thereby preventing toxicity to the photoreceptors [21,22]. Besides, fresh blood does not require special treatment, allowing for easy intraoperative observation. After coagulation, blood forms a complex with ILM that is not easily detached and can effectively seal the MH. Lai et al. [18] utilized autologous blood clots (ABC) assisted inverted ILM to treat HM MH with retinal detachment, achieving a retinal attachment and MH closure rate of 96% (26/27) after a single surgery. Autologous serum (AS), derived from the patient's own blood, offers excellent biocompatibility and contains essential growth factors (EGF, TGF-β, PDGF, fibronectin, vitamin A) while eliminating rejection risks, making it valuable for ophthalmic applications. In vitreoretinal surgery for ILM flap techniques, surgeons commonly use perfluorocarbon liquids (PFCL) despite risks of intraocular retention and higher costs, or ophthalmic viscosurgical devices (OVDs) which require specialized formulations and add expenses, whereas the autologous blood clot (ABC) technique provides a cost-effective alternative with reliable flap fixation and minimal complications, requiring only proper training to avoid temporary vitreous opacity, ultimately offering the best balance of efficacy, safety and cost-efficiency for ILM flap procedures. In our temporal group, no complications of flap displacement were observed. This finding further confirms that ABC can effectively stabilize the ILM flap and ensure surgical success. In our classic group, one case of flap displacement may have been due to HM accompanied by macular atrophy, which reduced visibility. Moreover, one case of ILM flap loss was observed in the classic group, and two cases exhibited flat/open closure following surgery. We propose that although ABC has been effective in fixing the ILM flap, the classic technique requires 360-degree loosening of the ILM, resulting in a narrower pedicle width. Furthermore, HM eyes with macular atrophy may reduce visibility during surgery, increasing the risk of ILM flap loss or displacement.
Michalewska et al. [9] conducted a prospective randomized controlled study on cases of idiopathic MH greater than 400 µm, comparing the classic inverted ILM flap technique (43 eyes) with the temporal inverted ILM flap technique (44 eyes). The initial BCVA for the two groups was 0.95 and 1.02, respectively (P = 0.28). There was no significant difference in visual acuity between the two groups during postoperative visits. However, both groups exhibited significant visual improvement after surgery (P < 0.001). In the classic group, the average BCVA improved to 0.66 logMAR at 3 and 6 months after surgery and further improved to 0.4 logMAR at 12 months. The visual acuity measured at 3, 6, and 12 months after surgery in the temporal group was 0.63, 0.68, and 0.45 logMAR, respectively. Ko ç ak et al. [11] reviewed 60 eyes with a minimum basal diameter greater than 600 µm, comparing ILM dissection and the temporal inverted ILM flap technique. The average improvement rate of BCVA was significantly higher in the temporal inverted ILM flap group compared to the ILM dissection group. Ho TC et al. [23] conducted a 12-month follow-up study on 18 patients with HM who underwent the temporal inverted ILM flap technique. The preoperative BCVA logMAR was 1.7 ± 0.6, and the postoperative BCVA logMAR was 0.72 ± 0.4 (P < 0.001). Takai et al. [13] retrospectively analyzed 11 cases of HM-associated MH treated with the temporal inverted ILM flap technique, with a follow-up period exceeding 6 months. The study confirmed a significant improvement in BCVA following surgery using the temporal valve technique (P < 0.05). Our results are consistent with previous studies demonstrating that both groups experienced improvement in postoperative vision, and there was no statistically significant difference between the two groups before and after surgery. However, a statistically significant difference in BCVA was observed in the temporal group before and after surgery. In contrast, the classic flip coverage group did not demonstrate a statistically significant change in visual acuity before and after surgery. We analyzed several possible reasons for these findings. First, the classical group had a higher incidence of complications, such as gliosis and valve loss. Second, the ILM flap in the classic group consisted of multi-layers, forming a plug-like structure that could hinder EZ/ELM repair. Third, patients with HM accompanied by macular atrophy may have had limited improvement in vision.
Both the temporal inverted ILM flap technique and the classical method achieved similar anatomical results in the treatment of MH, and the treatment effect for large-diameter holes is superior to ILM flap removal. In the randomized controlled study by Michalewska et al. [9], all hiatuses achieved closure following surgery, although not after the first surgery. The U-shaped closure rate in the classic group (62%) was lower than that in the temporal group (71%), though the difference was statistically non-significant. Twelve months postoperatively, the EZ defects in both groups decreased to 57%, while ELM defects were reduced to 24% and 25%, respectively. There was no statistically significant difference between the groups. In the study by Koçak et al. [11], the closure rate after a single surgery was 96.4% higher in the temporal inverted ILM flap group compared to the ILM dissection group (75.0%) (P = 0.029). Additionally, the U-shaped closure rate in the temporal inverted ILM flap group was 67.9% higher than that in the ILM dissection group (15.6%) (P < 0.001). At 6 months postoperatively, the overall recovery rate of EZ/ELM in the temporal inverted ILM flap group was significantly higher than that in the ILM dissection group (P=0.001).
The classic inverted ILM flap technique is often challenging to perform in patients with HM, long axial length, or coexisting macular atrophy. In contrast, the temporal inverted ILM flap technique is simple to operate and less prone to complications such as flap loss. The currently reported temporal flap method for treating HM-associated MH or MHRD has achieved high rates of retinal anatomical reduction and MH closure. Takai et al. [13] retrospectively analyzed MHs in HM (n = 11) and reported a 100% closure rate following a single surgery using the inverted ILM flap technique. Similarly, Ho et al. [23] studied the treatment of HM-associated MHRD using the temporal inverted ILM flap technique. Their study found that 17 eyes (94.4%) achieved retinal fixation through a single surgery, while 18 eyes (100%) completed fixation at the end of follow-up. Besides, all 18 eyes exhibited MH closure after complete gas absorption. The recovery of the macular structure was observed in 94.4% (17/18) of cases, where 14 eyes (77.8%) achieved full recovery of the ellipsoidal region, while 3 (16.7%) eyes exhibited partial recovery.
Consistent with previous studies, our study also achieved good closure rates for large-diameter MHs and HM MHs. Although there was no statistically significant difference between the two techniques in terms of EZ/ELM repair rate after surgery, the U+V closure rate of the temporal group was higher than that of the control group. We believe this is related to the fact that the ILM flap in the temporal group has a single-layer structure. In contrast, the classic group has multiple layers of ILM and may form a plug-like structure. The folding of a multi-layer ILM flap may hinder the rearrangement of the outer layer of the retina [24] and may also lead to excessive gliosis. The classic inverted ILM flap technique reported in the literature has a higher rate of glial cell proliferation (23%) [25]. Furthermore, even without intentional insertion, multi-layered inverted ILM flaps may migrate to the bottom of the hole under gas pressure, contributing to macular glial hyperplasia. There are limited reports on the gliosis rate associated with the temporal inverted ILM flap technique. In our study, no patients in the temporal group exhibited significant gliosis, whereas 3 cases (20%) of gliosis were observed in the classic group. Although the incidence of gliosis in the classical group was lower than reported in the literature, it remained higher than that in the temporal group. Consequently, we believe that the temporal ILM flap technique may offer advantages in reducing gliosis.
Flap closure refers to a distinct anatomical healing pattern observed after macular hole (MH) repair using the inverted internal limiting membrane (ILM) flap technique, characterized by:
Mechanical closure achieved solely by the inverted ILM flap acting as a "cover" over the defect, absence of complete neural retinal tissue regeneration beneath the flap, persistence of sub-flap hyporeflective spaces or outer retinal layer discontinuities on optical coherence tomography (OCT). A study suggests that 14% to 16% of eyes treated with an inverted ILM flap technique for FTMH may observe flap closure. KAROLINA BONI ´NSKA et al. believed that the additional retinal tissue under the flap would recover over time. From the third month on, flap closure was no longer observed. The eyes under investigation showed U-shaped, irregular and V-shaped foveal contours. Compared with the eyes that initially developed U-shaped, V-shaped or irregularly closed, the final vision of the eyes with flap closure was lower. The macular holes in the flap closure group were significantly larger than those in the group without flap closure. This study did not classify the eyes with flap closure: high myopia and non-high myopia [19]. In our study, one patient in the temporal group experienced flap closure that persisted for more than 3 months without any subretinal retinal tissue repair. We speculate that due to abnormal eye morphology caused by staphyloma, some single-layer ILM tissues did not touch the bottom of the MH, resulting in poor local stent formation and unrepaired retinal tissue. For HM MH, the intraoperative use of heavy water or multi-layer ILM may better facilitate ILM flap attachment.
Previous studies have reported that retinal thinning occurs following ILM peeling [26,27]. Aditya Modi et al. [27] observed significant thinning of the retina at a distance of 1.5 mm on the outer side of the macula after ILM peeling (P < 0.01). This study suggests that ILM detachment is associated with significant changes in the internal structure of the retina, which can have adverse effects on the functional outcome of surgery. Consequently, it is essential to avoid ILM detachment over larger areas. Reducing the area of ILM dissection without affecting the surgical outcome makes the temporal flap technique a better option. Chou et al. [15] found that in MH treatment using the temporal flap method, significant retinal thinning was observed in the lower outer quadrant of the temporal side, which was negatively correlated with BCVA. One explanation for this thinning is the damage to Mu¨ller cell footplates, which are more abundant on the temporal side [28]. Another explanation is related to retinal displacement. The thinning of the temporal retina and thickening of the nasal retina are both results of the displacement of the retina towards the optic disc during the closure of the hiatus [29,30].
In our study, both groups demonstrated a statistically significant decrease in retinal thickness within 1.5 mm of the macular center. This is related to preoperative MH edema and hole edge lifting, postoperative edema subsiding, and re-application of hole edge. The classic group exhibited similar results to previous studies, with retinal thinning occurring 1.5–3 mm above, below, and on the temporal side of the macula (P < 0.05). The change in retinal thickness on the lateral side of the nose was statistically non-significant, but the mean retinal thickness also decreased compared to preoperative levels.
The average retinal thickness of 1.5–3 mm outside the macular fovea in the temporal group exhibited a slight decrease, but this change was statistically non-significant. The temporal ILM flap technique that reduces the area of ILM removal appears to minimize the impact on retinal thickness. This may contribute to reducing the adverse outcomes of postoperative macular function. The comparison of retinal thickness between the two groups before and after surgery revealed a statistically significant difference only in the lower and outer regions. We speculate that it may be related to the folding and coverage of the ILM flap due to gravity.
ABC combined with the temporal inverted ILM flap technique offers a superior strategy for managing large-diameter or highly myopic macular holes (MH). It presents a less complex procedure than the traditional ILM flap method, with a markedly lower risk of flap displacement, ensuring robust visual and anatomical outcomes. This approach leverages the single-layer nature of the temporal inverted ILM flap to promote enhanced foveal morphological recovery while minimizing effects on retinal thickness. Importantly, the achieved “flap closed” state demonstrates stability for more than three months.
Our study has several limitations that warrant consideration. First, the modest sample size may limit statistical power, particularly for detecting small effect sizes. Second, as a retrospective study, we encountered typical challenges including missing data and potential selection bias. Third, the non-standardized follow-up protocols could introduce attrition bias, though we mitigated this through comparison with prior published reports. These limitations suggest our findings should be interpreted cautiously, and future prospective studies with larger cohorts and standardized protocols would be valuable to confirm our observations. In the future, multiple methods are needed to detect the results of temporal ILM flap technique, including microperimetry, etc.
Funding
No funds, grants, or other support was received.
Conflict of Interest Declaration
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
This retrospective study was conducted in accordance with institutional guidelines. The Ethics Committee of our hospital confirmed that ethical approval was not required for this research.
- Arda H, Maier M, Schultheiss M, Haritoglou C (2024) Advances in management strategies for large and persistent macular hole: An update. Surv Ophthalmol. 69: 539-46.
- Wakely L, Rahman R, Stephenson J (2012) A comparison of several methods of macular hole measurement using optical coherence tomography, and their value in predicting anatomical and visual outcomes. Br J Ophthalmol. 96: 1003-07.
- Michalewska Z, Michalewski J, Adelman RA, Nawrocki J (2010) Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 117: 2018-25.
- Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, et al. (2013) Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 156: 125-31.
- Mahalingam P, Sambhav K (2013) Surgical outcomes of inverted internal limiting membrane flap technique for large macular hole. Indian J Ophthalmol. 61: 601-3.
- Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J (2014) Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 34: 664-9.
- Kaluzny JJ, Zabel P, Kaluzna M, et al. (2021) Macular sensitivity in the area of internal limiting membrane peeling in eyes after pars plana vitrectomy with the temporal inverted internal limiting membrane flap technique for a full-thickness macular hole. Retina. 41: 1627-34.
- Tadayoni R, Svorenova I, Erginay A, Gaudric A, Massin P (2012) Decreased retinal sensitivity after internal limiting membrane peeling for macular hole surgery. Br J Ophthalmol. 96: 1513-16.
- Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J (2015) Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: A comparative study. Retina. 35: 1844-50.
- Shiono A, Kogo J, Sasaki H, et al. (2019) Hemi-temporal internal limiting membrane peeling is as effective and safe as conventional full peeling for macular hole surgery. Retina. 39: 1779-85.
- Kocak N, Yeter V, Birinci H (2023) Comparative study of conventional internal limiting membrane peeling versus temporal inverted internal limiting membrane flap for large macular hole treatment. Indian J Ophthalmol. 71: 188-94.
- Avci R, Mavi YA, Yilmaz S (2021) Evaluation of inner retinal dimples and internal limiting membrane flap configuration after temporal inverted ILM flap technique. Eur J Ophthalmol. 31: 649-55.
- Takai Y, Tanito M, Sugihara K, Ohira A (2019) The role of single-layered flap in temporal inverted internal limiting membrane flap technique for macular holes: Pros and cons. J Ophthalmol. 2019: 5737083.
- Stopa M, Ciesielski M, Rakowicz P (2023) Macular hole closure without endotamponade application. Retina. 43: 688-91.
- Chou HD, Chong YJ, Teh WM, et al. (2021) Nasal or temporal internal limiting membrane flap assisted by sub-perfluorocarbon viscoelastic injection for macular hole repair. Am J Ophthalmol 223: 296-305.
- Shin MK, Park KH, Park SW, Byon IS, Lee JE (2014) Perfluoro-n-octane-assisted single-layered inverted internal limiting membrane flap technique for macular hole surgery. Retina. 34: 1905-10.
- Lai CC, Hwang YS, Liu L, et al. (2009) Blood-assisted internal limiting membrane peeling for macular hole repair. Ophthalmology. 116: 1525-30.
- Lai CC, Chen YP, Wang NK, et al. (2015) Vitrectomy with internal limiting membrane repositioning and autologous blood for macular hole retinal detachment in highly myopic eyes. Ophthalmology. 122: 1889-98.
- Boninska K, Nawrocki J, Michalewska Z (2018) Mechanism of "flap closure" after the inverted internal limiting membrane flap technique. Retina. 38: 2184-89.
- Hirata A, Mine K, Hayashi K (2021) Contractility of temporal inverted internal limiting membrane flap after vitrectomy for macular hole. Sci Rep. 11: 20035.
- Benner JD, Hay A, Landers MR, Hjelmeland LM, Morse LS (1994) Fibrinolytic-assisted removal of experimental subretinal hemorrhage within seven days reduces outer retinal degeneration. Ophthalmology. 101: 672-81.
- Glatt H, Machemer R (1982) Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 94: 762-73.
- Ho TC, Ho A, Chen MS (2018) Vitrectomy with a modified temporal inverted limiting membrane flap to reconstruct the foveolar architecture for macular hole retinal detachment in highly myopic eyes. Acta Ophthalmol. 96: e46-e53.
- Abdul-Kadir MA, Lim LT (2021) Update on surgical management of complex macular holes: a review. Int J Retina Vitreous. 7:75.
- Iwasaki M, Miyamoto H, Imaizumi H (2020) Effects of inverted internal limiting membrane technique and insertion technique on outer retinal restoration associated with glial proliferation in large macular holes. Graefes Arch Clin Exp Ophthalmol. 258: 1841-49.
- Seo KH, Yu SY, Kwak HW (2015) Topographic changes in macular ganglion cell-inner plexiform layer thickness after vitrectomy with indocyanine green-guided internal limiting membrane peeling for idiopathic macular hole. Retina. 35: 1828-35.
- Modi A, Giridhar A, Gopalakrishnan M (2017) Spectral domain optical coherence tomography-based microstructural analysis of retinal architecture post internal limiting membrane peeling for surgery of idiopathic macular hole repair. Retina. 37: 291-8.
- Distler C, Dreher Z (1996) Glia cells of the monkey retina--II. Muller cells. Vision Res. 36: 2381-94.
- Akahori T, Iwase T, Yamamoto K, et al. (2018) Macular displacement after vitrectomy in eyes with idiopathic macular hole determined by optical coherence tomography angiography. Am J Ophthalmol. 189: 111-21.
- Ishida M, Ichikawa Y, Higashida R, Tsutsumi Y, Ishikawa A, et al. (2014) Retinal displacement toward optic disc after internal limiting membrane peeling for idiopathic macular hole. Am J Ophthalmol. 157: 971-7.

Tables at a glance