Edible Mushroom Significantly Ameliorated Hepatorenal Toxicity of Butyl Paraben (BP) In Albino Rats
Received Date: January 26, 2022 Accepted Date: February 26, 2022 Published Date: February 28, 2022
doi: 10.17303/jpam.2022.2.101
Citation: Kassahun Berhane (2022) Edible Mushroom Significantly Ameliorated Hepatorenal Toxicity of Butyl Paraben (BP) In Albino Rats. J Pathol Allied Med 1: 1-5.
Abstract
Introduction: Parabens are used commonly as preservatives in a range of cosmetics applied to the under arm and breast area as well as popular preservatives because of their cost.
Aim of the work: This study was done to evaluate the neprohepatic toxicity of parabens.
Materials and methods: Thirty adult female rats were used and given paraben orally for six months at parabens at dose of 10 % of the LD50 equal to 4.6mg\kg.bw. Mushroom was given orally to at dose of 10 mg/kg/day for six months too.
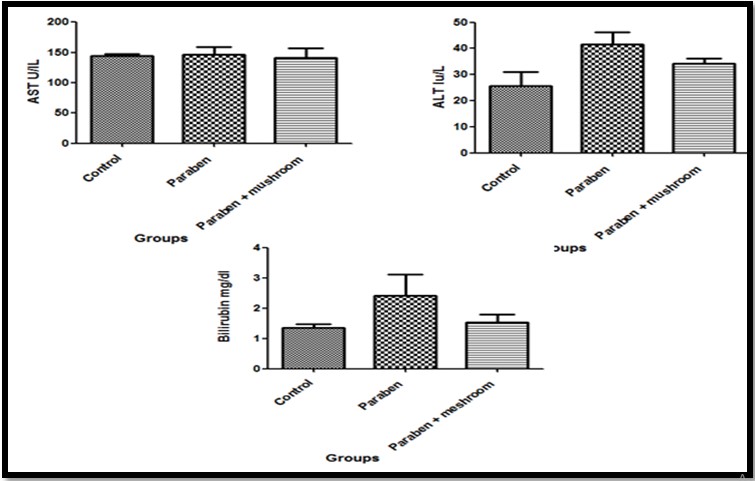
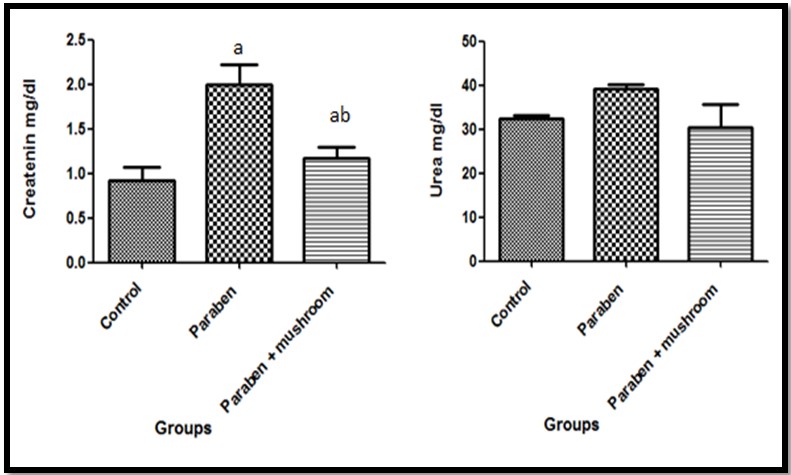
Results: Oral administration of BP induced biochemical and histopathological changes. Biochemical changes: BP toxicity manifested by changes in the liver and kidney function tests manifested by increase AST, ALT, Bilirubin, urea and createnine with decreases to plasma proteins in comparison to control group. Giving mushroom caused amelioration to the nephrohepatic toxicity by inducing recovery in liver and kidney functions in comparison to paraben treated group. For histopathological findings: BP induced vascular congestion in liver and kidney in association with necrotic changes in the hepatorenal epithelium which improved after mushroom treatment.
Conclusion: BP induced hepatorenal toxicity which improved by mushroom treatment.
Keywords: Butyl Paraben; Mushroom; Toxicity; Biochemical; Histological changes
Introduction
The term paraben (BP) is a mutual name of alkyl- or aryl-esters (chain length ranging from methyl- to n-butyl, isobutyl or benzyl) of parahydroxybenzoic acid (4-hydroxybenzoic acid, PHBA), an essential and ubiquitous plant constituent in cereals, fruit, vegetables or spices. PHBA is thought to be a common defense of plants against bacterial or fungal infections. For example, plant roots, such as carrots, were reported to contain up to 800 μg/gram dry mass of PHBA (Sircar et al., 2007). Parabens were used in pharmaceuticals since 1920 as single compounds or in combination to exert an antimicrobial effect (Sabalitschka, 1930). Parabens are popular name in the commercial industry as they are used daily in the products for humans and all humans are exposed to the BP compounds in one way or the other (e.g. food products, cosmetics powders and the pharmaceuticals preservatives materials (Fransway et al., 2019). The paraben products are stable at wide range of temperature and effective over high scale of pH values. BP has anti-bacterial, anti-fungal and anti-microbial properties (Alam et al., 2014; Abbas et al., 2010). They are soluble in water and oils to give a powerful fixative for liquefied phase as well as they have no noticeable odor and taste. Humans are primarily exposed to this compound via processed food like jams, jellies, canned fruits and beverages etc. (Boberg et al., 2016). Dermal application of cosmetic products like lipsticks, eye shadows, perfumes, foundations etc. are also one of main sources of absorption of this compound in human body. As females are more exposed towards cosmetic products so their exposure to this compound is greater in comparison to males (Giulivo et al., 2016). Liver is the main detoxification center of body; it plays an important role in production of anti-oxidants and maintains the metabolic mechanism of body. Raised production levels of AST, ALP and ALT are critical indicators of liver abnormality caused due to any exogenous or endogenous factor like toxic compound or virus etc. (Abbas et al., 2010; Boberg et al., 2010). Kidney normally excrete metabolic waste products (e.g., Body metabolites, drugs toxins byproducts) thus acute kidney injury is associated with retention of creatinine, urea, and other waste products (Mehat et al., 2007). BP is known to cause damaging effects on oxidizing organs like liver and kidney. BP may cause increased number of free radicles in hepatic cells and may lower the concentration of glutathione, superoxide dismutase and ascorbic acid (Watkins et al., 2015) [1-7].
Mushrooms are macrofungus with a distinctive fruiting body, which can be either hypogenous or epigeous, large enough to be seen with the naked eye and to be picked by hand (Chang et al., (1992). which considered a popular food source due to their flavors and textures and some spices also offer health benefits through supporting the immune system and thus lowering the risk of disease. Benefits include anti-carcinogenic properties as well as controlling the levels of glucose and blood lipids (Wong et al., ( 2008 (.Mushrooms are an excellent source of both proteins and carbohydrates while being low in fat, as well as providing minerals and free amino acids. In addition, they contain relatively large amounts of dietary fiber such as chitin and beta-glucans and are important sources of physiologically beneficial bioactive substances including polysaccharides, polyphenols, flavonoids, terpenoids and volatile organic compounds (Rathore et al., (2017).Protective mechanism exerted by mushrooms against toxicity was due to the anti-oxidant effects against ROS which coming through its beneficial bioactive compounds that they act as scavengers to the free radical species in the tissues (Soheir et al., 2007). Most commonly these BP compounds are absorbed inside body by oral intake and dermal applications. They are metabolized inside body by liver enzymes and byproducts are excreted out in bile and urine (Nowak et al., 2018). So this study was done to investigate the BP effects on the liver and kidney with the protective role of edible mushroom [8-11].
Materials and Methods
Experimental animals
Thirty adults female Wistar albino rats (with average weight about 200-250 gm and about two months old) were purchased from laboratory Medicine Animal House, Alborz, Iran and maintained in clean environment. The study protocol was approved by the Animal Ethics Committee at laboratory Medicine Institute, Iran. All animals were allowed to acclimatize in plastic cages (5 animals/ cage) inside a well-ventilated room for one week prior to the experiment. The animals were maintained under standard conditions (temperature of 23± 3ºC, relative humidity of 60–70%, and a 12 hour light/dark cycle), fed a diet of standard commercial pellets, and given water ad libitum.
Chemicals
N-butyl paraben (Butyl 4-hydroxybenzoate) N-butyl paraben (n-ButP, purity ≥ 99%, white crystalline powder) of analytical grade was purchased from Sigma Aldrich co., 3050 Spruce Street, St. Louis, and MO63103 USA.
b-Mushroom The fruiting bodies of mushroom (Pleurotus. ostreatus) were purchased from local market, Alborz, Iran.
c-Biochemical Kits The biochemical kits were used for determination of AST, ALT, Bilirubin, Total Protein, Albumin, Globulin, Creatinine and Urea. The kits were purchased from Alborz Laboratories, Iran.
Methods
Preparation of mushroom extract
The fruiting bodies of the mushrooms were cleaned and washed using tap water. Then, they were drained and cut into approximately equal-sized small pieces. After that, they kept in dry and clean place for dryness away from direct sun heat to avoid effect on nutrient content of the plant. The dried mushrooms were ground into fine powder using porcelain mortar and kept in air-tight plastic bags at room temperature for further processing. The dried mushroom powders were extracted using the methods of Lee et al., (2007).
For hot water extraction, 10 g of each mushroom powder was extracted with 100 mL of boiling water (100 ºC) and stirring at 150 rpm for 30 min. Then, the extracts were centrifuged at 5000×g for 30 min and the supernatants were filtered through Whatman's No.1 filter paper. The residues were re-extracted with one additional 100 mL portion of boiling water. The combined filtrates were evaporated to dryness under reduced pressure at 40 ºC using a rotary evaporator and this product was then further dried using a freeze dryer. The dried extracts were stored at - 18 ºC for further analysis.
Experimental design
Thirty adult female Wister albino rats were divided into 3 groups (n=10).Group 1 (control group): The rats were maintained as control group and supplied orally with 0.2 ml pea nut oil (vehicle). Group 2 (Paraben group): The rats were supplied daily orally with 10 % LD50 (4.6 mg/rat/day) dissolved in 0.2 ml pea nut oil. Group 3 (Paraben + Mushroom group): The rats were supplied daily orally with 10% LD50 equal to (4.6 mg/rat/day) dissolved in 0.2 ml pea nut oil according to Masten & Tice, (1999), plus 2 mg/rat/day (10mg/kg/day, Wu, et al., (2013) ) mushroom extract dissolved in 0.3 ml saline. Pea nut oil, Paraben and Mushroom were given orally using stomach tube for six months.
All animals in all groups were examined daily along the experiment and clinical signs were noticed. At the end of the experiment all rats were sacrificed. Blood samples were collected from the heart puncture in clean plan tubes. The serum was separated by centrifugation for 15 minutes at 3000 rpm and collected in labeled Eppendorf’s tubes and stored at -20 C until used for biochemical analysis by spectrophotometer (Bain et al., 2016). Tissue samples were taken from liver and kidney for histopathological examination [18-20].
Determination of liver and kidney function tests (LFT, KFT)
Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) were estimated according (Reitman and Frankel, 1957). Total Protein estimated according (Gornal et al., 1949). Albumin was determined according (Doumas et al., 1971). Globulin was determined by subtraction the value of the albumin from the value of the total protein according to (Howard, 1937). Creatinine was determined according to (Teitz, 1986). Urea was detected according (Tiffany et al., 1972) [21-23].
Histopathological examination
Specimens from liver and kidney of the sacrificed animals were collected, then fixed in 10% neutral buffered formalin, dehydrated in ethyl alcohol different concentrations (70%,80%,90%,95% &100%). Then, they embedded in paraffin blocks. Finally, sections about 5μm thickness were prepared by microtome and stained with Harries hematoxylin and eosin for histopathological examination, (Drury and Willington, 1980) [24].
Statistical analysis
Statistical analysis was done using one-way analysis of variance (ANOVA). It was done to compare between control and other treated groups, followed by post-hoc analysis (Dunnett's test) using SPSS (Statistical Package for Social Sciences) version 17 according to (Borenstein et al; 1997). Data were presented in the form of mean ± Standard Error. The difference was considered statistically significant when p < (0.05).
Results
Biochemical findings
Liver function test
Butyl paraben when given orally it induced changes in the liver function parameters. The liver enzymes activity was increased non significantly in comparison to control animals. The changes in the liver enzymes were associated with increase in serum bilirubin, total protein, albumin and globulin. The inoculation of mushroom induced recovery in the liver function parameters. Additionally mushroom treatment induced significant increase in total protein when compared with both control and paraben treated groups as shown in (Figures 1 & 2).
Kidney function test
In the kidney administration of butyl paraben for six months induced significant increase in creatinine when compared with control group while urea increased non- significantly. The increased in the creatinine was induced by butyl paraben was significantly reduced when mushroom inoculated as described in (Figure 3).
Histopathology
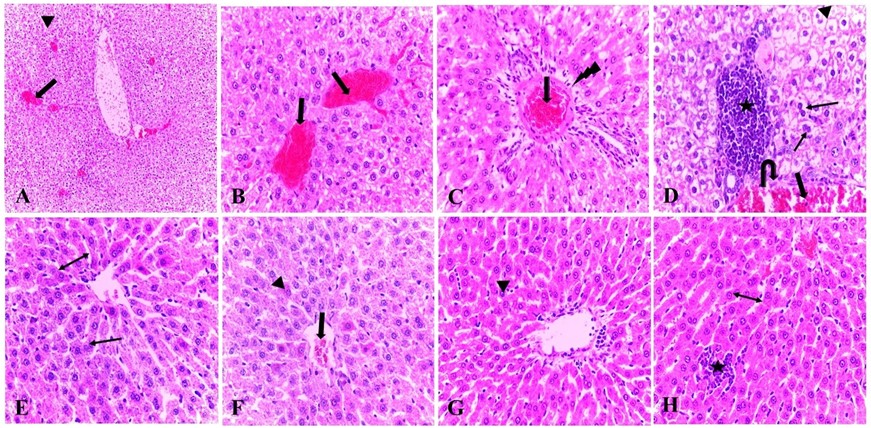
Histopathological lesions of livers treated with butylparaben were severe vascular congestion in the central and portal veins, prominent vacuolar degeneration associated with mild hepatic coagulative necrosis, severe periportal inflammatory reaction, biliary epithelial degeneration, endothelial cells lining the portal vein displayed necrosis, high mitotic frequency, dilated sinusoids, some cases revealed prominent hepatic necrosis and periportal edema. Liver treated with butylparaben and mushroom revealed amelioration in hepatic congestion, mild hepatic vacuolar degeneration, disappearance of inflammatory reaction in some cases, and others showed mild inflammation (Figure 4). Regarding the kidneys treated with butylparaben, the histopathological lesions were severe vascular congestion associated with interstitial leukocytic cell infiltration, glomerular necrosis, necrotic tubular epithelium, some of them showed degeneration leading to narrowing of the tubular lumen and many glomeruli revealed mesangial cell proliferation with congested blood capillaries. Kidneys treated with butylparaben and mushroom displayed restoration of urinary tracts and glomerular structure, most of the nuclei of the epithelial cells lining renal tubules were normal without any features of degenerations or necrosis, most of the epithelial lumen were opened and mild mesangial cell proliferation and leukocytic cell infiltration (Figure 5).
Discussion
In present study we reported biochemical and histological changes with pure butyleparaben toxicity; increase the liver enzymes activity of AST and ALT with decrease in the total protein, globulin and albumin in butyleparaben exposed group. These results are also reported by Darbre et al. (2008). Increased concentrations of hepatic enzymes and decrease plasma protein which were recorded in the current study were due to the action against the harmful effect of butyl paraben as these enzymes are accountable for many metabolic reactions in the hepatic cells. They assist in detoxification of poisonous compounds and also confer antioxidant mechanism. This is similar to a lot of studies that state, due to any exogenous or endogenous factor like toxic compounds or viruses, hepatic enzymes balance is disturbed (Abbas et al., 2010).
In case of kidney, urea and creatinine concentrations in serum of BP group were high as compared to (BP+AD) and control groups and it showed improper glomerular filtration in kidneys. These findings were corroborating with observations of Wahlang et al. (2013). As bilirubin levels were high due to the malfunctioning of hepatocytes to remove the metabolites of heme, this also effected the working ability of kidney cells and glomerular filtration rate, so resulted in high levels of urea and creatinine. In normal conditions creatinine is produced as a metabolite of creatinine and is processed by kidney and excreted out by urine but when kidneys are not working properly due to any exogenous and endogenous factors, its concentration gets increased (Harvey et al., 2006).
Histopathological finding of this study revealed degeneration and necrosis in hepatic cells in butyl paraben treated group. Hepatic portal vein was also damaged with congestions similar effects of butyl paraben were shown in study by Martin et al. (2010). On the other side when butyl paraben was co administered with mushroom, regenerative or protective potential of mushroom was cleared. Another study also proves the positive impact of mushroom on liver. According to that study damaged epithelial layer was refined to great extent by the active antioxidant effects of mushroom (Soheir et a.,l 2012). Malfunctioning of renal cells was also indicated by disrupted and changed epithelial boarders of glomerular cells. Debris are seen in peripheral and distal tubules along with necrotic and disruptive cells in glomerulus and bowman’s space and showed alike results to the findings of Verma et al. (2007). In butyleparaben and mushroom treated group, glomerular filtration was improved because renal cells showed recovery from toxic effects of butyleparaben and necrosis of cells was also decreased.
However, the combined administration of Mushroom and mushroom significantly reduced the increased values of the studied parameters (ALT, AST, Bilirubin, Creatinine and Urea) and decreased plasma proteins (total protein, albumin, and globulin) as well as the histopathological changes in liver and kidney were significantly improved by mushroom administration. This suggests that mushroom may be able to protect against the oxidation of hepatic and renal cellular membrane damage via a free-radical scavenging property (Nada et al., 2010). Soheir et al. (2012) demonstrated that the mushroom extract is able to significantly alleviate the hepatorenal toxicity induced by Ochratoxin in the albino rats. The protective mechanism of Mushroom may also involve enhanced immunity and downregulation of key cytokines (Vetvicka and Yvin, 2004). Moreover, Mushroom fruit bodies are rich with vitamins (B1, B2, C and D2), polysaccharides and glycoproteins (such as chitin, hemicelluloses, b- and a-glucans, mannans, xylans and galactans); all these contents have strong antioxidant, radical scavenging and other beneficial biological activities (Manzi et al., 1999; Mattila et al., 2000; Synytsya et al., 2009) [30-42].
Conclusions
The results of various parameters were synchronized, providing valid proof of butyl paraben's hepatic and renal toxicity in the above conditions, as well as the efficacy of mushroom as a hepatoprotective and nephroprotective agent.
Acknowledgement
Popularization of Rheology Science Program (PRSP); Project “Iranian Reality: The sustainability of scientific research during the Covid-19 pandemic.
- Abbas, S., Greige-Gerges, H., Karam, N., Piet, M.H., Netter, P., and Magdalou, J., 2010. Metabolism of parabens (4-hydroxybenzoic acid esters) by hepatic esterases and UDP-glucuronosyltransferases in man. Drug Metab. Pharmacokinet., 25: 568-577.
- Alam, M.S., and Kurohmaru, M., 2014. Disruption of Sertoli cell vimentin filaments in prepubertal rats: an acute effect of butylparaben in vivo and in vitro. Acta Histochem., 116: 682-687. https://doi.org/10.1016/j.acthis.2013.12.00
- Bain, B. J., Bates, I., & Laffan, M. A. (2016). Dacie and Lewis Practical Haematology E-Book. Elsevier Health Sciences.
- Bancroft, J. D., & Gamble, M. (Eds.). (2008). Theory and practice of histological techniques. Elsevier health sciences.
- Boberg, J., Axelstad, M., Svingen, T., Mandrup, K., Christiansen, S., Vinggaard, A.M., and Hass, U., 2016. Multiple endocrine disrupting effects in rats perinatally exposed to butylparaben. Toxicol. Sci., 152: 244-256.
- Boberg, J., Taxvig, C., Christiansen, S., and Hass, U., 2010.b Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol., 30: 301-312.
- Borenstein, M., Rothstein, H., & Cohen, J. (1997). Sample power statistics 10. SPSS Inc. Chicago.
- Chang, S. T., & Miles, P. G. (1992). Mushroom biology new discipline. Mycologist, 6(2), 64-65.
- Darbre, P.D., and Harvey, P.W., 2008. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol., 28: 561-578.
- Doumas, B. T., Watson, W. A., & Biggs, H. G. (1971). Albumin standards and the measurement of serum albumin with bromcresol green. Clinica chimica acta, 31(1), 87-96.
- Drury, R. A. B., and Wallington, E., A. (1980). Carleton's Histological Technique, 5th edt. Oxford University Press. New York.
- Fransway, A.F., Fransway, P.J., Belsito, D.V., and Yiannias, J.A., 2019. Paraben toxicology. Dermatitis, 30: 32-45
- Giulivo, M., De Alda, M.L., Capri, E., and Barceló, D., 2016. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. Rev. Environ. Res., 151: 251-264.
- Giulivo, M., De Alda, M.L., Capri, E., and Barceló, D., 2016. Human exposure to endocrine disrupting compounds: Their role in reproductive systems,metabolic syndrome and breast cancer. Rev. Environ. Res., 151: 251-264.
- Goldberg, D. M., and Spooner, R. J. (1983). Method of Enzymatic Analysis (Bergmeyen, H.V.Ed.) 3rd edn. Vol 3, pp258-265, Verlog Chemie, Deerfield beach, FI.
- Gornal A.C; Bardawill C.J.and David M.M.(1949 ) : J. Biol. Chem. 177 : 751
- Harvey, P.W., and Everett, D.J., 2006. Regulation of endocrine-disrupting chemicals: critical overview and deficiencies in toxicology and risk assessment for human health. Best Pract. Res. Clin. Endocrinol. Metab., 20: 145-165.
- Houwen, B. (2002). Blood film preparation and staining procedures. Clinics in laboratory medicine, 22(1), 1-14.
- Howard, F. E. (1937). U.S. Patent No. 2,095,095. Washington, DC: U.S. Patent and Trademark Office.
- Lee, Y. L., Yen, M. T., & Mau, J. L. (2007). Antioxidant properties of various extracts from Hypsizigus marmoreus. Food chemistry, 104(1), 1-9.
- Manzi, P., L. Gambelli, S. Marconi, V. Vivanti, L. Pizzoferrato, 1999. Nutrients in edible mushrooms: an inter-species comparative study. Food Chem., 65: 477- 482.
- Martín, J.M.P., Peropadre, A., Herrero, Ó. Freire, P.F., Labrador, V., and Hazen, M.J., 2010. Oxidative DNA damage contributes to the toxic activity of propylparaben in mammalian cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis, 702: 86-91.
- Mattila, P., K. Suonp V. Piironen, 2000. Functional properties of edible mushrooms. Nutrition, 16: 694-696.
- Mehat RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, et al (2007). Acute kidney Injury Network: Report of an initiative to Improve Outcomes in Acute kidney iinjury.Crit, Care 2007: 11:r31
- Nada, S.A., M.S. Arbid and R.R. Marquardt, 1994. Protective effect of L-cysteine on ochratoxicosis in rats. J.Egyp. Soc. Toxicol., 12: 33- 40.
- Nowak, K., Ratajczak–Wrona, W., Górska, M., and Jabłońska, E., 2018. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol., 474: 238-251.
- Nowak, K., Ratajczak–Wrona, W., Górska, M., and Jabłońska, E., 2018. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol., 474: 238-251.
- Pollock, T., Weaver, R. E., Ghasemi, R., & deCatanzaro, D. (2017). Butyl paraben and propyl paraben modulate bisphenol A and estradiol concentrations in female and male mice. Toxicology and applied pharmacology, 325, 18-24.
- Rathore, H., Prasad, S., & Sharma, S. (2017). Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition, 5(2), 35-46.
- Reitman, A. and Frankel, S. ( 1957) : Amer J. Clin. Path., 28 : 56.
- Sabalitschka, T. (1930). Ztechr. f. angew. Ch. 42, 936 (1929). Schachkeldjan, A.: Ztechr. f. anal, 139.
- Sircar, D., & Mitra, A. (2009). Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota 2: Confirming biosynthetic steps through feeding of inhibitors and precursors. Journal of plant physiology, 166(13), 1370-1380.
- Soheir Ahmed Al-Masri, Soha. M.S.El - Safty, Somaia A. Nada and Hassan A. Amra (2012). Hepato-renal Protective Effect of Edible Mushroom on Ochratoxin A Toxicity in Sprague Dawley Rats. Australian Journal of Basic and Applied Sciences, 6(8): 534-539, 2012 ISSN 1991-8178
- Synytsya, A., K. Mickova, A. Synytsya, I. Jablonsky, J. Spevacek, V. Erban, E. Kovarikova, J. Copikova,2009. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohyd.Polym., 76: 548-556.
- Tiffany TO, Jansen JM, Burtis CA Overtion JP, Soccot CD, 1972. Enzymartic Kinetic Rat and End point Analyses of Substrate by use of Gemsaec fast Analyzer. Clin chem .18:829-840.
- Titez, N W 1986: textbook of clinical chemistry.WB Saunders, Philadelphia pp, 1271 – 1281.
- Verma, R. J., and Asnani, V., (20070. Ginger extract ameliorates paraben induced biochemical changes in liver and kidney of mice. Acta Pol. Pharm., 64: 217-220.
- Vetvicka, V., J.C. Yvin, 2004. Effects of marine b-1,3 glucan on immune reactions. Int. Immunopharmacol.4: 721-730.
- Wahlang, B., Beier, J.I., Clair, H.B., Bellis- Jones, H.J., Falkner, K.C., Mcclain, C.J., and Cave, M.C., 2013. Toxicant-associated steatohepatitis. Toxicol. Pathol., 41:343-360.
- Watkins, D.J., Ferguson, K.K., Del Toro, L.V.A., Alshawabkeh, A.N., Cordero, J.F., and MEEKER, J.D., 2015. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int. J. Hyg. Environ. Health, 218: 212-219.
- Wong, K. H., & Cheung, P. C. (2008). Sclerotia: emerging functional food derived from mushrooms. Mushrooms as functional foods, 111, 146.
- Wu, X., Zeng, J., Hu, J., Liao, Q., Zhou, R., Zhang, P., & Chen, Z. (2013). Hepatoprotective effects of aqueous extract from lingzhi or reishi medicinal mushroom Ganoderma lucidum (higher basidiomycetes) on α-Amanitin− induced liver injury in mice. International journal of medicinal mushrooms, 15(4).



Figures at a glance