Covid-19 and Pathology Laboratory Safety Practice in Africa: Survey Results
Received Date: March 25, 2023 Accepted Date: April 25, 2023 Published Date: April 28, 2023
doi: 10.17303/jpam.2023.3.104
Citation: Eshetu L Haile, Melissa Kelly, Danny A Milner (2023) Covid-19 and Pathology Laboratory Safety Practice in Africa:Survey Results. J Pathol Allied Med 3: 1-11
Abstract
Background: Safety in the pathology laboratory is a pillar in laboratory procedures. Although histology laboratory inactivates many viruses through heat and chemical exposure, some practices pose a risk of infection transmission, thereby endangering staff, environment, and population safety.
Objective: This study was to understand to what extent a pathology laboratory in Africa could implement extra safety laboratory practices and measures during the COVID-19 pandemic.
Methods: A cross-sectional online survey was conducted on pathology laboratory safety practices in Africa between August 5-31, 2020.
Results: A total of 22 pathology laboratories participated in the survey, with 21 of them providing complete data. Most laboratories (90%) conducted FNA-associated activities in open-air laboratories. The average number of samples per month received before and during the COVID-19 pandemic was 423 and 168, respectively, a 60% reduction. More than eighty-five percent of pathology laboratories received non-fixed or fresh samples at the time of the COVID-19 pandemic, and 67% of them reported that facemasks and filter respirators were faced a shortage. Most pathology laboratories (85.7%) were not processing pathological samples in a BSC II during the COVID-19 pandemic.
Conclusion: Good pathology laboratory safety practices and personal protective equipment (PPE) devices should be in place. Special considerations of safety include re-designing the workflow, providing safety training, availing PPE, applying all safety practices, receiving fewer specimens, limiting the number of laboratory staff, and shifting program structure were presented as a solution during the Covid-19 pandemic. A larger sample size and more comprehensive survey could provide a better understanding of the implementation of laboratory safety practices during the COVID-19 pandemic.
Keywords: COVID-19; Coronavirus; Anatomic Pathology; Histopathology; Laboratory; Quality; Management; SLIPTA;ISO 15190
Introduction
Coronavirus disease 19 (COVID-19) is originated in Wuhan city of China in early December 2019. Currently, over 258 million coronaviruses cases are detected more than 5 million deaths are reported in 219 countries. In Africa,the number of cases has reached more than 8 million,with more than 222,276 deaths and 8,046,244 people were recovered [1,2].
Amid the challenges posed by the pandemic are concerned about addressing safety in pathology laboratories,which is a pillar in laboratory procedures and medical practice. Safety of personnel, environment, and product are influenced by the laboratory procedures, facility design, process flow and layout, the use and access of Personal Protective Equipment (PPE), and the level of biosafety. It is crucialto adhere to directives, policies, and procedures that ensure that all staff, the community, society, and the environment are protected [3,4].
Considering the fragility of laboratory facilities in Africa settings, with partial or not fully addressed of laboratory policies, laboratory safety practice, engineering control,administrative control, process flows and biosafety level, it'snecessary to use basic PPEs properly to protect themselves, families, community, and society at large in preventing the spread of the Covid-19 virus [3].
Histology laboratory receives various types of specimens like blood, urine, saliva, sputum, body fluids, organs,tissues, and cells to analyze and interpret. It mainly analyses tissue’s and cells’ shapes, sizes, and architectural patterns.Laboratory safety applies to all three phases of pre-, analytical,and post-analytical processes that include tissue acquisition,slide, and test preparation, communication, and reporting [3-5].
Fixed tissues and paraffin blocks are low risks of coronavirus infection; however, laboratory staff faced a challenge when manipulating unfixed and inadequately fixed samples that require strict adherence to biosafety rules [6-7].
Although pathology laboratories analyze human tissues and cells for diagnostic, therapeutic, prognostic, and forensic, fortunately, routine histology processes inactivate many viruses through heat and chemical exposure [8-10].Frozen tissues sectioning may be a possible risk of COVID-19 unless the laboratory specifies aerosol prevention and containment procedures. Most pathology laboratories are receiving samples of unknown status [8,11].
Conducting a laboratory safety survey is paramount to obtaining insight into pathology laboratory safety practice during the Covid-19 pandemic in Africa. The study aimed to understand to what extent a pathology laboratory in Africa could implement extra safety laboratory practices and measures during the COVID-19 pandemic.
Methods
Ethical considerations: Our study did not involve samples of human subjects or animals. The study rather asked voluntary laboratories to participate in the online survey.This study didn’t request pathology names, locations,addresses, or identification codes to gain the required information.The only identification system was used an email address, and we kept it confidential. The informed consent was collected that indicate their willingness prior to joining the survey call; however, if they were not willing to participate, they could withdraw or refuse anytime. Study population and sampling strategy: The online survey link was shared to 22 pathology laboratories in Africa by the American Society of Clinical Pathology (ASCP) to fill up the survey voluntarily. Respondents have been working in Anatomic Pathology (AP) laboratory in Africa, and the majority of them are pathologists.
Questionnaires: Forty-nine (70%) of survey questionnaires were closed-ended questions, and 40 of them had two or three options “Yes”; “Partially” and “No”. Furthermore,eight questions had more than three multiple choices.A total of 21 questions were open, and 5 and 11 of them requested the respondents to answer the “Why” and “What”questions; respectively, the rest explained the situation.
Data collection: The survey poster described project rationale, objective, procedures, anonymity, confidentiality,voluntary participation, questionnaire, and filling instruction.A single response per laboratory is allowed and,to limit multiple submissions, the study used an automatic popup dialog box: “No additional submission allowed”. The estimated time of online survey completion was 10-15 minutes.The primary survey data found at https://docs.google.com/forms/d/1q_3qimZjyrE_qpVpFDg Hc9EpikDrbRxgeojiwge9wXM/edit?gxids=7757
Data analysis
Data were entered, cleaned, checked for validity,and analyzed. Descriptive statistics such as percentage,count, and pie graph were used.
Results
A total of 22 pathology laboratories participated,and 21 of them completed the survey (a dataset excluded due to missing variables). Thirteen of the 21 pathology laboratories (61.9%) were supported by the American Society for Clinical Pathology (ASCP), Center for Global Health,Cancer diagnosis, and treatment. Most participating pathology labs were public (81.0%), and others were public-private partnerships (PPP, 9.5%) and private pathology laboratories (4.8%).
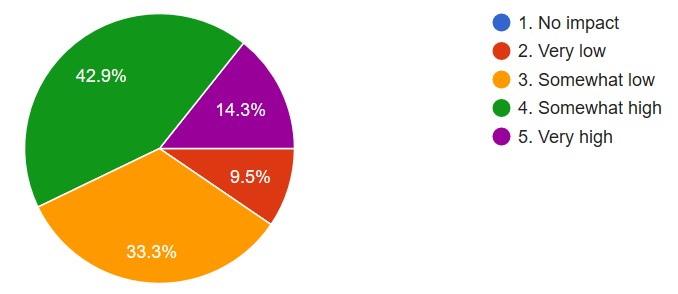
The impact of COVID-19 on daily pathology laboratory performance (scale measurement: “no impact“ to“very high impact”) was 57.2% documented (Figure 1). The respondents described the following specific reasons why the performance was affected:
The number of surgical procedures reduced; some staff got infected that lead to lost working hours, and staff were afraid to come for work.
- The number of clients attending the FNAC clinic becomes low.
- The hospital designated as a reference center for the COVID-19 and was entirely dedicated to caring COVID-19 patients.
- The income of path labs drops three times than previous, and adversely the expenses rise by about 60%.
- Delays in delivery of purchased reagents like gloves, masks, and virucide reagents.
- Increased risk of COVID-19 exposure of the patients, pathology staffs, and other personnel.
- Reduction in number of samples processed, staff work in shifts to ensure social distancing, the reluctance of some staff to handle specimens and lab reports, irregular supply of reagents and repair of equipment due to lockdown restrictions.
- The pathology lab is next to the mortuary,and the COVID-19 cases brought directly without giving them any protective measures except the surgical mask. The pathologist was still performing the post-mortem without knowing the patient's status. The COVID-19 bodies can stay in there for more than three days.
- Due to personal movement restriction,there is a remarkable reduction in patient flow to our hospital. Especially the cytology (FNAC) is affected significantly that reduced the request by more than 2/3”.
- Around 80% to 90% reduction in the incoming volume of both cytology and biopsy specimens. These have significantly impacted their activity, especially their residency program in anatomic pathology, necessitating a change to the academic calendar.
Specimen Handling
The average number of samples received per month before and during the COVID-19 pandemic were decreased significantly from 423 to 168, 60.3% reduction found. Despite the low volume of pathology samples, routine pathological testing continued in many labs (85.7%,18/21) with a minimum of four working hours and a maximum of 24 hours opened.
Most pathology labs (85.7%, 18/21) were not processed pathological samples in a Biosafety Cabinet (BSC II) during the COVID-19 pandemic. The labs revealed that the main reasons for these were:-
- Left the samples in formalin for more than 48 hours
- Lack of funds
- Not available, processing goes the same way as before COVID
- They have just an exhaust fan in the grossing area
- The lab ordered the equipment; however,it took longer to receive
- They are practicing pathology operations in open-air laboratory rooms
The pathology laboratory (85.7%, 18/21) received non-fixed samples like cytology or large fresh organ tissue specimens during the pandemic. Moreover, samples like non-gynecological and Fine Needle Aspirate (FNA) were accessioned and processed on open-air lab workstations that accounted for 81% of the laboratories (17/21), and only 9.5% (2/21) processed samples inside a fume hood.
Workload and Staffing
More than half of the pathology labs (52.4%) did not reduce staff number at the time of the pandemic, and the rest (33.3%) reduced their staff, and others applied various mechanisms such as reduced working hours (4.8%),working hours shifts (4.8%), and forced employees to work on alternative days.
Safety Equipment
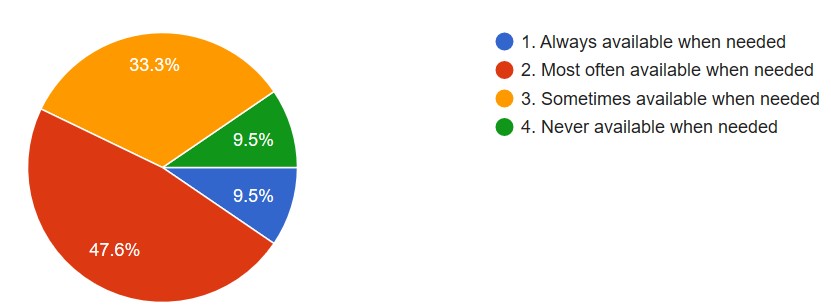
Ten laboratories (47.6%) confirmed that PPE was available, but 9.5% of them mentioned that PPE was never available when needed (Figure 2). However, 76.2% of labs encountered a shortage of safety supplies because of the COVID-19 pandemic. Fifty percent of them confirmed that extra safety precautions were not available for sample handling that potentially produce aerosols or droplets during aspirating fluids from the needle or syringe, smear preparation,and air or heat drying processes. Furthermore, 85.7% of the pathology labs never changed their technical procedures to fit into the COVID-19 situation.
Most of the respondents (76.2%) were changed their facemask or respirator (N-95) once per day, and 19.0% changed their facemask depending on lab workload or other conditions. There was a shortage of facemasks and filter respirators in most labs (67%) due to suppliers’ irregularity,non-availability in the entire country, price increased significantly, prioritization issues, budget constraints, and shortage in the market. Most respondents (71.4%) confirmed that they disinfected PPE and reused it again due to inaccessibility of supplies, cost, and non-availability.
Lab Services and Turnaround Time (TAT)
There was no room for switching to selective pathology tests,90.5%(19/21), during the COVID-19 situation;however, 9.5% of the laboratories experienced switching of service because of reduction surgical theater procedures and to avoid the risk of spread of COVID-19 among laboratory staff.
The average TAT of histology and cytology tests during COVID-19 reduced by 72.8% and 52.3%, respectively.The average TAT of histology test before and during COVID-19 pandemic was 9.2 and 2.5 days, and for cytology test, 8.6 days and 4.1 days, respectively. Most pathology labs (61.9%) did not change their lab workflow as a result of the COVID-19 pandemic; however, 38.1% of them experienced workflow change and adopted new work shifts (66.7%).Most laboratories (81.0%) revealed that no pathology lab services were interrupted during the COVID19; however,19.0% of labs had faced a complete or partial service suspension.
Sample/Slide Processing
Most pathology labs (90.0%) used air-and/or heat drying of smears in open-air, and the rest was processed in fume hoods. Furthermore, 61.9% of the laboratories indicated that pathological slides were handled or transferred without special safety measures during the COVID19 pandemic.However, the laboratories demarcated a “dirty” and “clean” workstation during the COVID-19 situation.
Half of the laboratories (50.0%) confirmed that they didn’t diagnose unfixed pathology slides in clean office spaces to avoid transmission of COVID-19 and to avoid a dangerous practice. However, 95.2% of the laboratories used standard sample fixation time and never changed their predefined time. And 76.2% of laboratories didn’t have special precautions when unfixed samples arrived. In contrast,some pathology labs arranged special safety precautions include:disinfecting the sample container with Sodium hypochlorite, heating lab result form (using an iron), changing the fixative, and increasing the volume of fixative; increasing fixation time, and using proper PPE.
Risk Assessment and Mitigation
Most of the laboratories (61.9%) didn’t conduct any risk assessment activities and implementation plans during COVID19. However, 15 pathology laboratories (71.4%) used an additional cleaning and disinfection program to reduce COVID19 associated risks and they received COVID-19 related lab safety training. One-third of labs (33.3%) revised their laboratory safety manuals or procedures during the COVID19 situation. Laboratories (95.2%) limited face-to-face personal meetings since COVID19 began,and more than 57.0% of staff were instructed or engaged in COVID19 specimen management. However,61.1% of the respondents indicated that no specific COVID-19 procedures were available for specimen receiving, handling, and testing.
Almost all laboratories (90.5%) revealed that adequate hand sanitizer and cleaning materials were available without constraint; however, 57.1% of the respondents stated that no additional safety practices were in place on tissue and body fluid waste management. This could be explained by laboratories never receiving a sample from a suspected case, treating the waste with formalin or alcohol, fixing it before incineration, handling only fixed histopathology specimens, using safety materials, or no supplies.
Most laboratories, 85.7% (18/21), were notified their staff not to enter to lab unless wearing surgical masks and washing their hands. The same percentage of laboratories instructed their staff to maintain an appropriate social distance between co-workers while operating in the laboratory.Nevertheless, 14.3% of the laboratories reported that not possible to maintain physical distance due to the narrow lab space and not being easy to implement in practice.
Autopsies
Over half of laboratories (57.1%) performed autopsies during the COVID19 pandemic. They did all necessary safety precautions by employing full PPE; using minimally invasive tissue sampling (MITS) instead of open autopsies;screening all autopsies for COVID-19 and getting PCR test results before postmortem.
Staff Belongings, Dress, and Break Rooms
Most respondents (71.4%) reported that they were never aware of or instructed about personal belongings like mobile phones, wearing jewelry, rings, and watches shouldn’t bring to the lab. Additionally, 57.1% of the respondents didn’t know about laboratory policy to confine or contain long hair and avoid wearing loose clothes. However, 66.7% (14/21) confirmed that the laboratory management had banned or prohibited personnel from wearing open-toed shoes, sandals, and footwear with holes on the top. Furthermore, 81% of the laboratories had no smoking, cosmetics application, drinking, eating and, gum chewing. Some laboratories,19.0%, didn’t follow Good Laboratory Practices (GLP), had no safety policy, and no staff restroom.
Discussion
Pathology laboratories in Africa faced several challenges,despite the labs are practicing extensively in dealing with biological and chemical hazards. The COVID-19 pandemic has brought significant effects on the performance of the laboratory and is forced to change the existed working culture into a new-normal cycle where the redesign of samplesflow, sample handling and accession, staffing, strict safety practice, work shifts, resources, facility layout, workflow,policy, and guidelines are necessary. For example, the restriction of personnel movement, the number of surgical procedures reduced, and the staff was afraid of infection leading to limited working hours [7,12,20].
Our findings suggested that in times of uncertainty like the Covid-19 pandemic need strong communication and collaboration platforms are needed for Africa to fill up the gaps timely in terms of developing and sharing specific pathology laboratory safety policy, guidelines, and infrastructure [5,11,13].
Most pathology laboratories didn’t have Biosafety Cabinet (BSC II) or fume hood to protect the personnel, environment,and product. A new strategic initiative may consider for the handling of samples when unfixed/partial/inadequate fixed, large fresh specimens, non-gynecological, and Fine Needle Aspirate (FNA) collected to avoid an open-air operation [14-16].
The authors emphasized that anatomic and surgical pathology laboratories didn't get the necessary attention.Strict safety management and reinforcement by the Ministry of Health and global partners are needed. Furthermore,a simplified procurement system for COVID-19 related supplies may design to cut the bureaucratic process.
Pathologists and laboratory scientists who remain forefront in handling biological tissues and samples are the risk of infection. And these may minimize by all means through testing and communicating Covid-19 results timely to make all necessary safety precautions followed [3, 16,17].
In Africa, a typical pathology laboratory didn't equip with high-state-of-the art technology where they operated in outdated machinery, which further aggravates the safety concerns. These practices may far violate the ideal way of processing specimens under a laminar flow hood [9,14,18].
According to the College of American Pathologists (CAP), the minimum turnaround time report sign-off for a routine histopathology laboratory is within two days or less (>=90%). However, in our study before the COVID-19 pandemic,the average TAT of histology sign-off did not meet the requirement. Interestingly, during COVID-19 the laboratories were achieved the minimum requirement due to the low flow of samples received. Therefore, the laboratory should redesign the system by deploying all necessary managerial and technical requirements to improve the turnaround time for routine cases [19,22].
The management of laboratory chemicals is a pillar for controlling and monitoring various hazards and toxic chemicals; however, a significant number of pathology labs didn’t manage their chemicals based on materials safety data sheet (MSDS) guidelines. The laboratory requires a chemical hygiene plan, environmental monitoring, employee education, facility engineering and administrative controls,process value engineering, PPE, chemical storage, and chemical inventory [3,20,21].
The management of unfixed, partially fixed, inadequate fixed specimens or slides could be the leading cause of infection transmission and may have a direct impact on workforce absenteeism. A system could design to avoid exposure for emerging and reemerging infection by transforming the laboratory into a state-of-art laboratory by deploying automatic fixation and processing of slides where a significant number of pathology labs in Africa examine unfixed/inadequate slides in the clean office spaces. CDC states that handling and testing of histopathology and surgical samples should be performed in a BSL-2 laboratory [5,6,16]. There are various safety-related standards used to curb laboratory safety malpractice such as OSHA (Occupational Safety and Health Administration) or ISO15190:2020 Medical laboratories- requirements for safety [12,21].
Most pathology laboratories didn’t have a system of monitoring and controlling hazardous permissible exposure limits where significant hazards and risks may be happening any time when working with unknown hazard substances,mixture, and unknown toxicity. According to OSHA standards, the permissible exposure limits (PEL) device and method should be in place [21].
In recent times, in Africa, laboratories have been accredited by independent third-party with ISO15189:2012. However, there is no special arrangement for a certifying pathology laboratory on safety [13]. Regional Office for Africa (World Health Organization) has developed a program called SLIPTA (Stepwise Laboratory Quality Improvement Process towards accreditation) which helps laboratories to assess safety-related gaps and allows improvement [15].
Pathology laboratory processes require a safety risk assessment, intending to eliminate hazards wherever possible. Where these cannot be possible, the risk from each hazard may reduce or transfer to as low a level as practicable,by order of priority, 1) substitution; 2) containment; or 3) using personal protective measures and equipment. The universal safety rule and principle in most African pathology labs have not been practiced as expected. A full-fledged safety risk assessment program should design and integrate into the regular laboratory program.
In Africa, laboratories thought that laboratory safety was not their primary consideration, but they suffered their health and works in due course. The primary goal of safety control programs in histopathology laboratories is to promote laboratory personnel and make the working environment safe. Sharing best laboratory safety practices and experience in the continent for a common goal in responding to current global health crises is required.
The study recommends that pathology laboratory samples may process at Biological Safety Cabinet II (BSC-II),fume hood, tissue grossing machine, or additional PPE devices to protect any forms of contamination between specimen,environment, and personnel. Safety policy and manuals should be in place, and an appropriate biosafety risk assessment program is also necessary in the case of Covid-19.During emerging or remerging infection, the pathology laboratory should always look at entire alternative safety cultures and practices, design new ways of doing things and strive for its effectiveness.
To mitigate the impact of the pandemic on pathology laboratory operations, there is a need for proactive measures such as contingency planning, stockpiling of critical supplies, and flexible staffing arrangements. Pathology laboratories should collaborate with hospitals and other healthcare providers to optimize patient flow and ensure continuity of care during the pandemic. Laboratories should explore innovative solutions such as tele pathology and digital pathology to minimize the need for in-person consultations and reduce exposure risks.
The authors suggest that there is a need for strong communication and collaboration platforms to develop and share specific pathology laboratory safety policies, guidelines,and infrastructure. It is also recommended that anatomic and surgical pathology laboratories receive necessary attention, and strict safety management is reinforced by the Ministry of Health and global partners.
The authors suggest that a system could be designed to avoid exposure to emerging and re-emerging infections by transforming the laboratory into a state-of-the-art laboratory by deploying automatic fixation and processing of slides. The study recommends that pathology laboratory samples may process at Biological Safety Cabinet II (BSC-II),fume hood, tissue grossing machine, or additional PPE devices to protect any forms of contamination between specimen,environment, and personnel.
Limitation
The reality and the performance of safety practices in the pathology laboratory may not be accurate. Moreover,the respondents may not gather information physically at the time of this study conducted. The authors were not able to observe the actual safety practice in their respective laboratories.
The study only included 22 pathology laboratories in Africa, which may not be representative of the entire African region. The study didn’t able to provide a detailed analysis of the challenges that laboratories faced in implementing laboratory safety practices. Therefore, future studies could explore the challenges in-depth and provide evidence-based recommendations to address the challenges. Furthermore,the study did not discuss the cost implications of implementing laboratory safety practices, particularly in low-resource settings.
Furthermore, the study relied on self-reported data,which may be subject to biases and inaccuracies. Future studies could consider expanding the sample size and using a mixed-methods approach that incorporates both quantitative and qualitative data collection methods to gain a more in-depth understanding of laboratory safety practices during the COVID-19 pandemic. Additionally, the study could also consider including a follow-up survey to assess whether any changes in laboratory safety practices occurred after the initial survey.
Conclusion
Good pathology laboratory safety practices and personal protective equipment (PPE) devices should be in place. Special considerations of safety include re-designing the workflow, providing safety training, availing PPE, applying all safety practices, receiving fewer specimens, limiting the number of laboratory staff, and shifting program structure were presented as a solution during the Covid-19 pandemic.
Moreover, it would be helpful to have more data on the potential risks of COVID-19 transmission in frozen tissue sectioning and the specific aerosol prevention and containment procedures that laboratories should implement to mitigate these risks. Further studies could also examine the impact of the COVID-19 pandemic on the workflow,staff, and program structures of pathology laboratories in Africa and identify strategies to address these challenges.
Funding
This study was funded by the American Society for Clinical Pathology, Center for Global Health.
Acknowledgements
The authors would like to thank the participants, despite dealing with the challenges and setbacks of COVID-19 that took the time to complete the survey. Sincere thanks and gratitude to the ASCP staff, members, and our governance for their support and guidance in our global health mission.
Author Contributions
Eshetu L Haile: involved in the design and write up of proposal, data collection and analysis, draft the manuscript, feedback for journal reviewers, manuscript edition;Danny A. Milner: involved in the proposal write up and manuscript edition, and funding the project; Melissa Kelly: involved in the proposal write up and manuscript edition.
Competing Interests
The author(s) declare that there is no financial or personal relationship(s) that may have inappropriately influenced us in writing this article.
Data availability statement
The data that support the findings of this study are openly available here and click: COVID-19 AND PATHOLOGY LABORATORY SAFETY PRACTICE IN AFRICA: ONLINE SURVEY - Google Forms
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
- COVID-19 Coronavirus Pandemic (October 2020).
- Kohn LT, Corriga JM, Donaldson MS, editors. Washington (DC): National Academy Press; 1999. To Err is Human: Building a Safer Health System.
- Iyengar JN (2009) Quality control in the histopathology laboratory: an overview with stress on the need for a structured national external quality assessment scheme. Indian J Pathol Microbiol 52: 1-5.
- Henwood A (2019) A survival guide for laboratory professionals.Scotts Valley, CA, USA: Amazon Create Space Independent Publishing Platform; Chapter 18 Disinfection 149-155.
- Centers for disease control and prevention, laboratory biosafety guidelines for handling and processing specimens associated with Coronavirus Disease 2019 (COVID-19).
- Henwood A.F (2020) Coronavirus disinfection in histopathology.J Histotechnol 1-3.
- Darnell ME, Taylor DR (2006) Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion 46: 1770-7.
- Guarner J (2020) Three emerging Coronaviruses in two decades the story of SARS, MERS, and now COVID-19. Editorial Am J Clin Pathol.
- Public Health England. 2020. COVID-19: safe handling and processing for samples in laboratories.
- Henwood AF (2018) Ebola and histotechnologists. J Histotechnology 41: 71-73.
- Soniya Adyanthaya, Maji Jose (2013) Quality and safety aspects in histopathology laboratory. J Oral Maxillofac Pathol 17: 402-7.
- "About Novel Coronavirus (2019-nCoV)". United States Centers for Disease Control and Prevention (CDC). 11 February 2020. Archived from the original on 11 February 2020. Retrieved 25 February 2020.
- Berte LM (2007) Laboratory quality management: a roadmap. Clin Lab Med 27: 771-90.
- Iwen PC, Stiles KL, Pentella MA (2020) Safety Considerations in the Laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome Coronavirus 2 (SARS- CoV-2). Am J Clin Pathol.
- WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) in the African Region (with checklist).
- World Health Organization (2020) Laboratory biosafety guidance related to coronavirus disease (COVID-19): interim guidance.
- European Centre for Disease Prevention and Control.Considerations related to the safe handling of bodies of deceased persons with suspected or confirmed COVID-19.
- Fineschi V, Aprile A, Aquila I et al. (2020) Management of the corpse with suspect, probable or confirmed COVID-19 respiratory infection-Italian interim recommendations for personnel potentially exposed to material from corpses, including body fluids, in morgue structures and during autopsy practice. Pathologica Epub.
- World Health Organization (2020) Guidance on COVID-19: Safe handling and processing for samples in laboratories.Public Health England.
- World Health Organization (2020) Guidance on regulations for the transport of infectious substances.
- Occupational Safety and Health Administration. Safety and Health Topics/COVID-19. 2020.
- Saeed Alshieban, Khaled Al-Surimi (2015) Reducing turnaround time of surgical pathology reports in pathology and laboratory medicine departments. BMJ Qual Improv Rep4: u209223.w3773.

Figures at a glance