Phytochemical Screening and Toxicity Studies of Aqueous Leaf Extract of Traditional Medicinal Plant Cistus ladaniferus (L). in Mice and Rats
Received Date: October 23, 2023 Accepted Date: November 23, 2023 Published Date: November 25, 2023
doi: 10.17303/jpdm.2023.6.101
Citation: Fadila Moussaoui, Tajelmolk Alaoui, Mohamed Ramchoun, Nesserine Bennani (2023) Phytochemical Screening and Toxicity Studies of Aqueous Leaf Extract of Traditional Medicinal Plant Cistus ladaniferus (L). in Mice and Rats. J Pharmacol Drug Metab 6: 1-12
Abstract
The purpose of this study is to evaluate the acute and sub-chronic toxicity of the aqueous leaf extract of Cistus ladaniferus (C. ladaniferus ) in rats and mice.
Acute toxicity was studied in mice distributed in several lots. The six batches of mice received intraperitoneal (ip) doses corresponding to 200, 400, 600, 800, 1600, 3200 mg / kg of body weight for 14 days. Control mice received 0.9% NaCl. For sub- -chronic toxicity the aqueous extract was administered orally to adult Wistar rats at different doses (500-1500 mg / kg / day, respectively, for 90 days). The animals are sacrificed. Blood samples are taken to study the parameters (hematological and biochemical) and the organs (liver, spleen, kidneys) are taken for histopathological analysis.
The results obtained show that the aqueous extracts of C. ladaniferus have a lethal dose LD50 of 831.76 ± 0.12 mg / kg of body weight. In all the parameters taken into account, no visible change was observed in the treated mice compared with the control groups. Sub-chronic toxicity results showed no clinical symptoms nor biochemical or histological alteration in rats treated.
The result indicates that intraperitoneal and oral administration of the aqueous extract of C. ladaniferus did not produce a significant toxic effect in mice and rats.
Keywords: Cistus Ladaniferus; Acute Toxicity Test; Sub-Chronic Toxicity Test; Aqueous Leaf Extract; Phytochemical Screening; Traditional Medicinal Plant
Abbreviations: (DL50), Dose médiane; (I.P), Intra-péritonéale; (EDTA), Ethylenediamine tetra-acetic acid; (Hb), hemoglobin; (HT), hematocrit; (RBC), red blood cells; (GB), blood cells; (PLAQ), platelets. (CRE), creatinine; (AST), aspartate aminotransferase; (ALAT), alanine aminotransferase; (NaCl), Le chlorure de sodium;(LDH), lactate dehydrogenase ;(ALP),alkaline phosphatase.
Introduction
Human societies have been in close contact with their environment since the beginning oftheir training and used the ingredients of the environment to obtain food and medicines [1]. Medicinal plants have historically proved their value as a source of molecules with therapeutic potential, and today still represent an important reservoir for the identification of new drug molecules [2].
Although herbal medicine to occupy a prominent place in the therapeutic arsenal, he use of medicinal plants is not insignificant, indeed, many plants are toxic and their misuse can be dangerous [3-5]. It is clear that in therapy, everything depends on the dose that remains a masterpiece in the therapeutic arsenal of medicinal plants. One of the problems of using herbal medicines is that in many cases, no defined doses are prescribed, often resulting in overdose [6]. C. ladaniferus is a plant belonging to the family Cisitaceae, represented by seven genera comprises 16 species, particularly prevalent in the Mediterranean [7]. C. ladaniferus is widespread in Portugal, Spain, Italy, Algeria, and Morocco [8]. It is a plant widely used in Western herbal medicine.
Studies have shown that C. ladaniferus has anti-- fungal [9], antiviral, anti-inflammatory, gastro protective, and antitumor effects [10]. Flavonoids and tannins are the main compounds of C. ladaniferus , they are widely appreciated for their potentially beneficial effects on health, as antioxidants [11,12]. The aqueous extract of C. ladaniferus has antidiarrheal and anti-propulsive activity [13]. It has also been considered as a curative and preventive treatment against hypertension [14].
In order to identify the toxicological aspect of C. ladaniferus, we proceeded to the determination of the lethal dose (LD50) allowing knowing the smallest dose which administered at one time causes death of 50% of the animals. This study also makes it possible to specify the clinical signs of intoxication and to define the limits of tolerance to the product. In addition to this irreversible toxicity, we have studied the risks of latent or non-apparent sub-chronic toxicity, which makes it possible to search for and characterize the functional and / or pathological changes caused by a long-term treatment, thus highlighting the possible effect of possible accumulation of the substance and detecting the target organs by histopathological examination.
Material and Methods
Plant Material
C. ladaniferus was harvested in the Khénifra region during the period May-June 2014. All samples were shaded before use. Botanical identification and authentication of the plant material was done by Ms Leila NASIRI, Professor of the Faculty of Sciences of Meknes, Morocco. The plant material was dried in the shade and protected from moisture at room temperature for a few days before being crushed and stored until use.
Preparation of plant extracts
30 g of the powder of the C. ladaniferus leaves were boiled in 300 ml of distilled water for 15 minutes. After cooling, the extract is concentrated on a rotary evaporator. The yield of the extraction is 13.3% relative to the dry plant.
Phytochemical screening
We proceeded to search for the main chemical groups present in our aqueous extract. These generally sought after chemical groups are: alkaloids, sterols, triterpenes, saponins and polyphenolic compounds (tannins, flavonoids, quinones). Phytochemical analysis was performed according to the methods described by [15-17].
Experimental Animals
Adult healthy Swiss mice weighting 20–30 g aged two months were used. These mice come from the breeding center of the Institut Pasteur, Casablanca, Morocco. For the sub-acute toxicity, albino Wistar rats weighing 170–260 g were used. These animals were provided by the Laboratory Animals of the Faculty of Sciences, University of Moulay Ismail, Morocco.All animals were fed with standard laboratory diets, given water ad libitum and maintained under laboratory conditions.
Acute toxicity tests
The animals were divided into seven experimental groups of 10 animals each (5 males and 5 females). The 1st group served as a negative control, while 2nd, 3rd, 4th, 5th and 6th was considered as tested groups received intraperitoneally C. ladaniferus extract at the doses of 200, 400, 600, 800, 1600, 3200 mg / kg, respectively.
Animals were observed individually for general behavioral (activity, gait, tremor ...), body weight changes and mortality during the first 30 min, 1, 2, 4, 6 hours and once daily for 14 days after administration of the extract.
Then the mice were sacrificed and their organs subjected to macroscopic observation [18]. The mortality is recorded 1, 2, 24 hours, then 2 and 3 days after taking the aqueous extract. The LD50 was calculated by the method of [19].
Sub-chronic toxicity study
In the sub-chronic study, Wistar rats in four groups of 8 animals (4 males and 4 females) for each dose level of C. ladaniferus were used in these tests. Group I was considered as control and the other two groups which were considered as tested groups received the plant extract at a dose of 500 and 1500 mg/kg body weight respectively for 90 days. Toxicity was evaluated in terms of corporal and organ weights, gross behaviour, haematological, biochemical characteristics and mortality. The organs (liver, spleen, kidneys) are removed, weighed and stored in Bouin's fluid for histopathological analysis [18,20,21].
Blood analysis
The blood sample was collected into ethylene diamine tetraacetic acid (EDTA) tubes for hematological and biochemical parameters. Sera samples were prepared by 10 min centrifugation of blood at 3500 rpm.
Histopathological study
After sacrificing the rats, the liver and the kidneys were removed and fixed in formalin. The next step is dehydration. This is usually done with a series of alcohols; say 70% to 95% to 100%.Each organ was sectioned, embedded in paraffin wax and then stained with hematoxylin-eosin and examined under a light microscope.
Statistical Analysis
All results are expressed as mean ± SD. The comparison of the groups with the control is carried out using the Primer of biostatistic software. P-values less than 0.05 were considered to be significant.
Results
Phytochemical Screening
The phytochemicals are Tannins, flavonoids and saponines presented in the aqueous leaf extract of C. ladaniferus (Table 1).
Acute toxicity study
In this part of the work, we undertook the study of the acute in vivo toxicity linked to the extract of Cistus ladaniferus, in order to determine the LD50, and to evaluate the clinical signs observed.
Preliminary tests of the aqueous leaf extract of Cistus ladaniferus, administered (IP) to mice at concentrations 200, 400, 600, 800, 1600, 3200 mg/kg, demonstrate the absence of toxicity of the plant extract up to the dose 400 mg/kg: 0% mortality after 14 days.
Note that from the concentration 600mg/kg, we observed clinical signs (tremors, hard stomach, accelerated breathing, rigidity of the hind limbs, loss of appetite, etc. (Table 2)
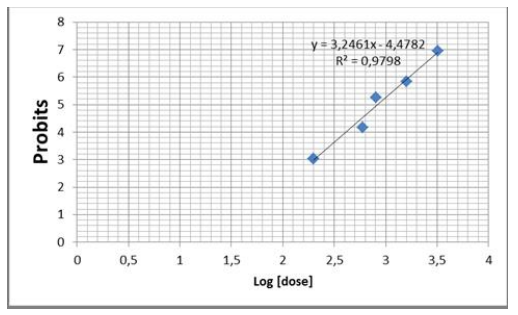
After intraperitoneal administration of the different doses (200, 400, 600,800, 1600, 3200 mg/kg), the LD50 is respected in mice at a dose of 831.76 ± 0.12 mg/kg (intraperitoneal route) (Figure1). Deaths of male and female mice are observed 36 and 48 hours after administration of the product.
On all the parameters taken into account, no visible change was observed in the treated mice compared to the control groups. According to our results, C. ladaniferus could be classified as low toxicity.
Sub-chronic toxicity study
Relative organ weight
Daily oral administration of the aqueous leaf extract of C. ladaniferus for 90 days did not produce any obvious symptoms of toxicity or mortality up to the highest dose level of 500 and 1500 mg / kg / day (Table 3).
The body and organs weight of the animals given C. ladaniferus extract once a day during 90 days did not show any significant differences compared with the control group.
Relative weight of liver, kidney and spleen
The effect of the extract on the weights of vital body organs is shown in Table 4. The body weight variation of the rats of both sexes in all test and satellite groups was statistically not significant compared to that of their controls. The results revealed that, the vital organs such as liver, kidney, heart and spleen were not adversely affected throughout the treatment by extract. Studies have shown that weight reduction of the body and internal organs is considered as an index of sensitivity to toxicity after exposure to a substance [22,23].
Hematological analysis
The effects of extract on hematological parameters are summarized in (Table 5). No significant difference was observed from the results of the hematological profile hemoglobin, hematocrit, red blood cells, white blood cells and platelets) of rats ofboth sexes. There were generally no significant differences noted between control and treated groups for the hematological parameters measured.
Biochemical analysis
The effects of repeated administration of C. ladaniferus extract for 90 days on serum biochemical parameters are summarized in Table 6.
All the tested biochemical parameters including Creatinine (CRE), aspartate aminotransferase (AST), alanine aminotransferase (ALAT), and total proteins were within normal limits compared to control group.
No significant changes were observed in the different biochemical parameters studied. Normal creatinine values suggest that this extract did not alter renal structure and function.In our study and following the treatment of the rats, no adverse effects were observed on the morphology of the kidneys, spleen and liver of the treated rats.
Histology of liver, spleen and kidney
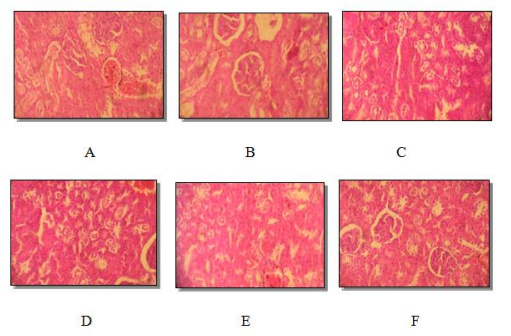
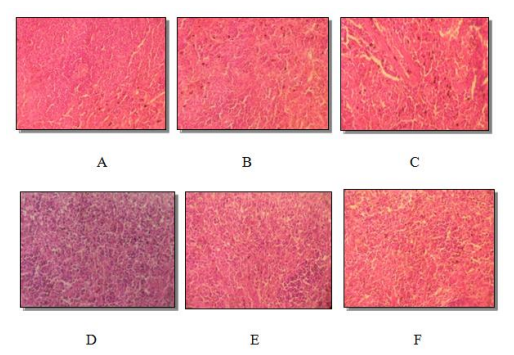
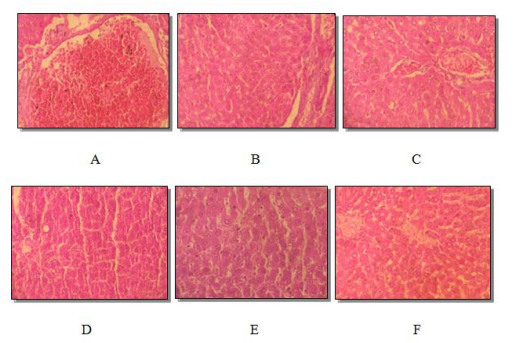
Histology of the kidney, spleen and liver sections of male and female rats who received C. ladaniferus extract at different doses for 90 consecutive days is presented in Figure2, Figure 3, and Figure 4. Microscopical analysis of the examined internal organs displayed normal aspect of the liver, kidney and spleen for the two doses used (500mg / kg and 1500 mg / kg). No significant differences were grossly detected between control and experimental groups.
Discussion
Toxicological studies of plants with therapeutic uses are today a key step in the process of valuing aromatic and medicinal plants.
Conventionally, in the presence of a substance endowed with biological activity, the study of the toxicity and in particular the evaluation of the LD50 proves essential. This value already gives an estimate of the toxicity of the studied substance and will be used to establish the therapeutic coefficient characterized by the ratio between the LD50 and the active dose.
Phytochemical analysis of the aqueous extract of C. ladaniferus revealed the presence of flavonoids, tannins and saponins.
Acute toxicity assessment allowed determination of LD50 in mice treated intraperitoneally with aqueous extract of C. ladaniferus. The value of the LD50 was estimated by the Miller and Tainter graphical method. The results obtained show that the aqueous extract of leaves of C. ladaniferus has a lethal dose LD50 of 831.76 ± 0.12 mg / kg.
According to the [24] classification, chemicals with an LD50 between 500 and 5000 and between 5000 and 15000 mg / Kg are considered almost moderately and non-- toxic, respectively. From this classification it can be concluded that the aqueous leaf extract of C. ladaniferus was classified as moderately toxic in mice. A study by [13] showed that the aqueous leaf extract of C. ladaniferus leaves is considered non-toxic. Another study by [9] showed that the aqueous leaf extract of C. ladaniferus is considered moderately toxic.
The evaluation of sub-chronic toxicity at doses of 500 and 1500 mg / kg / day by daily oral administration of the aqueous leaf extract of C. ladaniferus resulted in no death, no evidence of change in morphology, body weight and body weight. The behavior of treated animals is quite comparable to that of untreated rats. Studies have shown that reduction in body and internal organ weight is considered an index of sensitivity to toxicity after exposure to a toxic substance [22,23]. Our aqueous leaf extract of C. ladaniferus was also without effect on the biochemical and hematological parameters. Normal creatinine values suggest that this extract did not alter renal structure and function.
With respect to other biochemical parameters such as total protein no significant difference was observed compared to the control group. In addition, the transaminase enzymes SGOT (AST) and SGPT (ALT) were not disturbed, the liver was not reached. These parameters are not statistically different (p> 0.05).
In addition, that transaminases enzyme SGOT (AST) and SGPT (ALT) were not disturbed, the liver was not reached and the total proteins did not undergo variations during the study. These parameters are not statistically different (p> 0.05).
Transaminases, LDH, and alkaline phosphatases ALP are good indicators of liver, heart and kidney toxicity, respectively [25]. The microscopic observation of these organs shows a normal appearance of the kidneys of the rats and this for the two doses used (500mg / kg and 1500 mg / kg). The liver also has a normal appearance similar to that of control rats.
Histological sections of kidneys, spleen, and liver from treated rats appeared normal compared to control rats (Figure 2, Figure 3 and Figure 4). Hepatic tissue had liver lobules formed from normal hepatocyte cells in the male and female treated groups (Figure 4). Our results are in agreement with those found by [26,5].
The liver and kidneys are target organs for toxic chemicals because of their essential functions in body detoxification and excretion processes [26,27]. As well as the liver is considered an important organ in a toxicological study [28]. Histological features of nothing showed a normal structure of glomeruli and Bowman capsules (Figure 2). Thus, the morphology of the proximal and distal convoluted tubules shows no abnormality. Similar results have been reported by [5] in rats treated with Senna alata.
Histopathological examination of the spleen sections showed a normal splenic parenchyma with no abnormalities in the white and red pulp of both treated and control groups (Figure 3). Our results are in agreement with those found by [29]. The present study shows that daily oral administration for 90 days of the aqueous leaf extract of C. ladaniferus does not cause any mortality, nor alter the biochemical and histopathological parameters of the treated rats. Under these experimental conditions, the plant extract appears to be devoid of toxicity in male and female albino rats.
The toxicological study of medicinal plants is a substantial step in order to determine the limits of their tolerances and subsequently to optimize their use. The information from the set of results presented in this work on the experimental acute toxicity data in mice suggests that the aqueous extract of C. ladaniferus administered intraperitoneally is classified as a weakly toxic. However, sub-chronic toxicity shows that the aqueous extract of C. ladaniferus is completely devoid of toxic effects. In this sense, the rare work done on the toxicity of C. ladaniferus confirms the safety of the plant. This issue is currently of great interest and deserves to be widely exploited.
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
- Jamshidi-Kia F, Lorigooini Z, Amini-Khoei H (2018) Medicinal plants: Past history and future perspective. Journal of Herbmed Pharmacology 7: 1-7.
- Atanasov et al. (2015) Discovery and resupply of pharmacologically active plant-derived natural products: A review. Europe PMC Funders Group Author Manuscript Biotechnol Adv.Author manuscript; available in PMC 33: 1582–614.
- Balajis S, Chempakam B (2010) Toxicity prediction of compounds from turmeric (Curcuma longa L). Food Chem Toxicol 48: 2951-9.
- Santosh N, Mohan K, Royana S, Yamini TB (2010) Hepatotoxicity of tubes of indian Kudzu (Pueraria tuberrosa) in rats. Food Chem Toxicol 48:1066-71.
- Roy S, Ukil B, Lyndem LM (2016) Acute and sub-acute toxicity studies on the effect of Senna alata in Swiss Albino mice. Cogent Biology 2: 1272166.
- Usman MM, Sule MS, Gwarzo MY (2014) Toxicological studies of aqueous root extract of Euphorbia lateriflora (Schum and Thonn) in rats. Journal of Medicinal Plants Studies 2.
- Talavera S, Gibbs PE,Et Herrera J (1993) Reproductive Biology Of Cistus Ladaniferus (Cistaceae). Plant Syst. And Evol 186: 123-34.
- Mariotti JP, Tomi F, Casanova J, Costa J, Bernardini AF (1997) Composition of the Essential oil of Cistus ladaniferus L. Cultivated in Corsia (France), Flavour and Fragrance Journal 12: 147-51.
- Barros L, et al. (2013) Antifungal activity and detailed chemical characterization of Cistus ladanifer phenolic extracts. Industrial Crops and Products 41: 41–5.
- Skoric M et al. (2012) Cytotoxic activity of ethanol extractsof ethabol of in vitro grownCistus creticus subsp. Creticus L. on human cancer cell lines, industrical Crops and products 38 : 153-9.
- Amensour M, Sendra E, Pérez-Alvarez JA, Skali-Senhaji N, Abrini J, Fernández-López J (2010) Antioxidant activity and chemical content of methanol and ethanol extracts from leaves of rockrose (C. ladaniferus ). Plants Foods Hum. Nutr 65: 170-8.
- Guvenc A, Yildiz S, Ozkan AM, Erdurak CS, Coskun M, Yilmaz G, Okuyama T, Okada Y (2005) Antimicrobiological studies on Turkish Cistus species. Pharm. Biol 43: 178-83.
- Aziz M. et al. (2011) Relaxant effect of aqueous extract of Cistus ladaniferus on rodentintestinal contraction, Fitoterapia 77: 425-8.
- Belmoukhtar M, et al. (2009) Antihypertensive and endothelium-dependent vasodilator effects of aqueous extract of Cistus ladaniferus. Biochemical and Biophysical Research Communications 389: 145-9.
- Wagner H, Bladt S (1996) Plant Drug Analysis: A Thin Layer Chromatography Atlas. Berlin. Springer Science Business Media.
- Bekro YA, Bekro JAM, Boua BB, Tra Bi FH, Ehilé EE (2007) Etude ethnobotanique et screening phytochimique de Caesalpinia benthamiana (Baill.) Herend et Zarucchi (Caesalpiniaceae). Rev. Sci.Nat 4: 217-25.
- Trease E, Evans WC (1987) Phermacognosy. Billiare Tindall. London 13: 61-2.
- Kanjanapothi et al. (2004) Toxicity of curde rhizome extract of Kaempferia galanga L (Proh Hom). Journal of Ethno pharmacology.
- Miller LC, Tainter ML (1944) Estimation ofLD50 and its error by means of log-probit graph paper. Proc Soc Exp Bio Med 57: 261.
- Palmeiro NMS, Almeida CE, Ghedini PC, Goulart LS, Pereira MCF, Huber S, Silva JEP, Lopes S (2003) Oral subchronic toxicity of aqueous crude extract of Plantago australis leaves. Journal of ethno pharmacology 88: 15-8.
- Al Habori M, Al-Aghbari A, Al-Mamary M, Baker M (2002) Toxicological evaluation of Catha edulis leaves: a long term feeding experiment in animals. Journal of Ethno pharmacology 83: 209-17.
- Raza M, Al-Shabanath OA, El-Hadiyah TM, Al-Majed AA (2002) Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Scientia Pharmaceutica 70: 135-45.
- Thanabhorn S, Jenjoy K, Thamaree S, Ingkaninan K., Panthong A (2006) Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. Journal of Ethno pharmacology 107: 370-3.
- Loomis TA, Hayes AW (1981) Loomis’s essentials of toxicology. New York: Academic Press 1996.
- Martin DW, Mayes PA, Rodwell YM. In:Harper’s Review of Biochemistry. 18th edn, Lange Medical 61.
- Bello I, Bakkouri AS, Tabana YM, Al-Hindi B, Al Mansoub, MA, Mahmud R, Asmawi MZ (2016) Acute and Sub-Acute Toxicity Evaluation of the Methanolic Extract of Alstonia scholaris Stem Bark. Med. Sci 4: 4.
- Adekomi DA, Musa AA, Tijani AA, Adeniyi TD, Usman B (2011) Exposure to smoke extract of Datura stramonium leaf: Some of its effects on the heart, liver, lungs, kidneys and testes of male Sprague Dawley rats. Journal of Pharmacognosy Phytother 3: 67-75.
- Kalaiselvia P, Umaa M, Suresha M, Thulasiramana K, Lakshmidevib E (2013) Chronic toxicity studies of aqueous leaf extract ofIndian traditional medicinal plant Ocimum tenuiflorum (Linn.) in rats. European Journal of Experimental Biology 3: 240-7.
- Treadway S (1998) An ayurvedic herbal approach to a healthy liver. Clinical Nutrition Insights 6: 1-3.

Tables at a glance
Figures at a glance