Harnessing Melatonin and Herbal Medicine for Circadian Rhythm Modulation in Cancer Therapy
Received Date: April 15, 2024 Accepted Date: May 15, 2024 Published Date: May 18, 2024
doi: 10.17303/jpdm.2024.7.102
Citation: A. Tavartkiladze, G. Simonia, D. Kasradze, P. Revazishvili, L. Tavartkiladze, et al. (2024) Harnessing Melatonin and Herbal Medicine for Circadian Rhythm Modulation in Cancer Therapy. J Pharmacol Drug Metab 7: 1-17
Abstract
Melatonin, a naturally occurring hormone synthesized by the pineal gland during the dark phase of the circadian cycle, has garnered significant attention for its role in regulating circadian rhythms and its potential therapeutic applications in cancer treatment. Circadian rhythms, the internal clock that governs the physiological and behavioral processes in living organisms, play a critical role in maintaining homeostasis and have been increasingly implicated in the pathogenesis and progression of various cancers. This abstract outlines the multifaceted role of melatonin in modulating circadian rhythms, its therapeutic potential in cancer treatment, the significance of herbal medicine as a source of melatonin, and the emerging paradigm of circadian cancer therapy as a novel and promising treatment regimen.
The synthesis and release of melatonin are intricately linked to the body's circadian rhythms, serving as a physiological signal of darkness. It influences various biological functions, including sleep regulation, immune response, and antioxidant defense mechanisms. Beyond these roles, melatonin has been shown to possess oncostatic properties, making it a molecule of interest in cancer research. Its anticancer effects are attributed to its ability to induce apoptosis in cancer cells, inhibit tumor growth and proliferation, and enhance the efficacy of conventional chemotherapy and radiotherapy treatments. Moreover, melatonin's ability to regulate circadian rhythms suggests its potential in synchronizing the internal clock disrupted by cancer, thereby mitigating the adverse effects of circadian disruption on cancer prognosis.
Herbal medicine, a rich source of melatonin and other bioactive compounds, offers promising avenues for cancer prevention and treatment. Several herbs, including St. John's Wort (Hypericum perforatum), known for its high melatonin content, have been used traditionally for their medicinal properties. These herbs not only contribute to the exogenous supply of melatonin but also offer a synergistic combination of compounds that may enhance melatonin's therapeutic effects. The integration of herbal medicine into cancer treatment regimens highlights the importance of natural products in developing effective and holistic approaches to cancer therapy
The role of circadian rhythms in cancer therapy has emerged as a critical area of research, with evidence suggesting that the timing of cancer treatment according to the body's internal clock can significantly impact treatment outcomes. This approach, known as circadian cancer therapy, aims to optimize the timing of drug administration to coincide with periods of maximum efficacy and minimum toxicity, thereby improving the therapeutic index of anticancer agents. By aligning treatment schedules with the patient's circadian rhythms, circadian cancer therapy seeks to enhance drug effectiveness, reduce side effects, and improve the quality of life for cancer patients.
In conclusion, melatonin's regulatory effect on circadian rhythms and its oncostatic properties underscore its potential as a valuable adjunct in cancer therapy. The exploration of herbal medicine as a source of melatonin and other anticancer compounds further supports the integration of traditional knowledge into modern oncology. Moreover, the advent of circadian cancer therapy represents a novel treatment paradigm that leverages the intrinsic biological rhythms to maximize therapeutic efficacy and minimize toxicity. Together, these elements highlight the significance of circadian rhythms in cancer treatment and the potential of melatonin and circadian-based strategies as innovative approaches to combat cancer.
Keywords: Melatonin; Circadian Rhythms; Herbal Medicine; Circadian Rhythms in Cancer Therapy; Circadian Cancer Therapy
Introduction
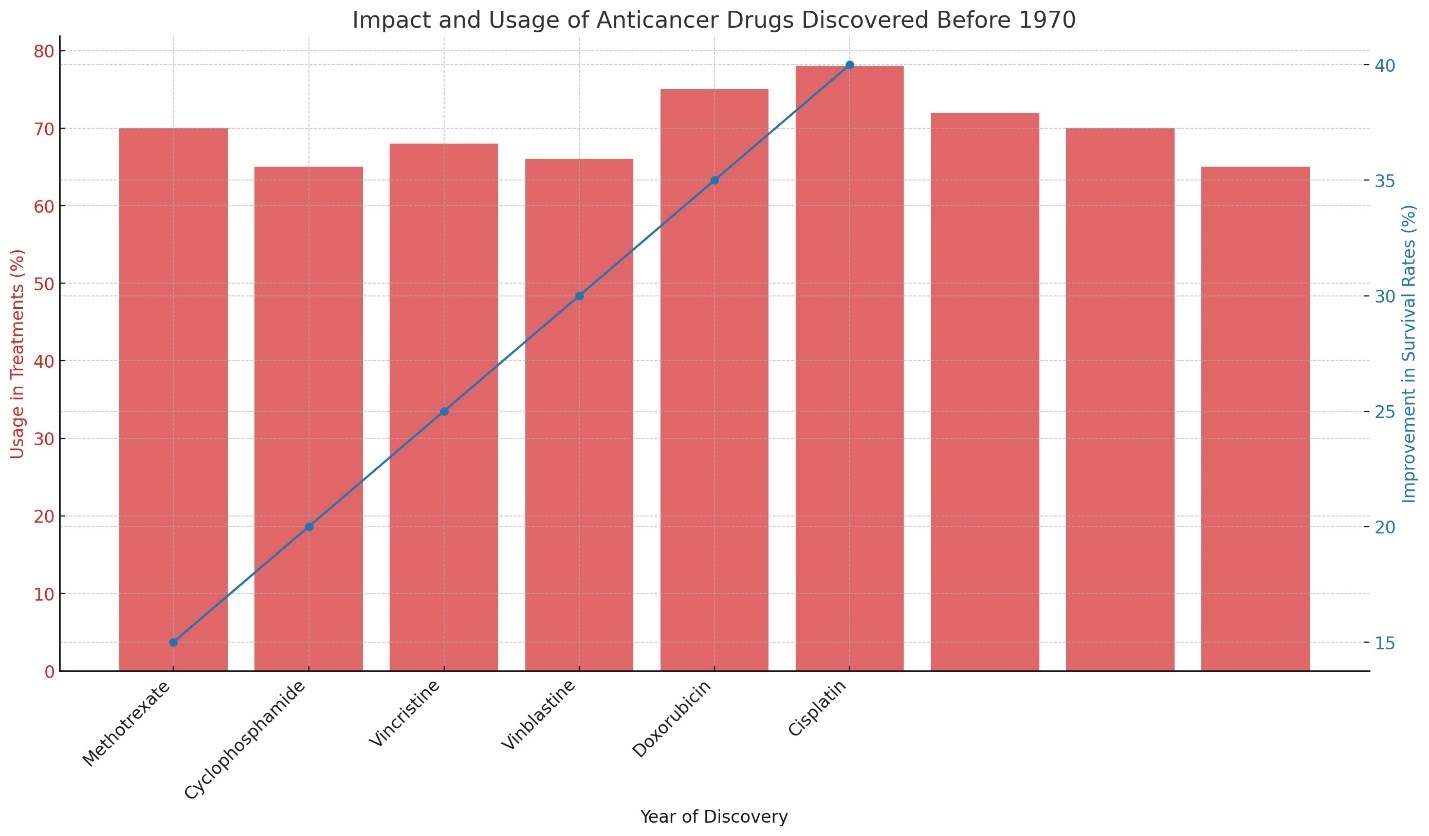
Malignant tumors are a significant group of diseases that cause nearly 19 to 20 million deaths worldwide each year. Various treatment strategies are employed to combat cancer, including surgery, chemotherapy, radiotherapy, and both immuno- and targeted therapies. However, the outcomes are often unsatisfactory [4]. Currently, anticancer drugs (cytostatics) developed before 1970, such as methotrexate (1949), cyclophosphamide (1950), vincristine (1961), vinblastine (1959), doxorubicin (1960), cisplatin (1965), carboplatin (1970), melphalan (1953), and 5-fluorouracil (1956), are used in up to 70% of chemotherapy treatments in clinical oncology [5]. This raises the question: Has nature exhausted its supply of discoverable substances, or has scientific oncology reached an impasse? Despite significant scientific progress in oncology, including targeted, immuno-, and gene therapies, and T-cell therapy, survival rates for cancer have only improved by 20% or not at all in 67% of cases, according to a 2022 report by the NIH and IARC [4]. Research from the Francis Crick Institute published in 2022 suggests that despite the influence of epigenetic factors and changes in environmental factors over the last 70 years, tumor cells should theoretically be resistant to cytostatics discovered before 1970, but this is not the case. In fact, cytostatics from before 1970 are seeing more use, have more pharmacological indications, and are yielding more positive results in clinical oncology than molecules developed after 1990 [6]. According to Chinese and Japanese biomedicine, we are entering an era of active use of biomolecules, indicating a return to nature and natural remedies (with judicious and academic application) to preserve health—not only in oncology. This is evidenced by the growing volumes of pharmacopeias from China, India, and Japan (Figure 1).
One of the key biocomponents is melatonin, an endogenous hormone linked to the regulation of the circadian rhythm, the most universal indicator of metabolism. All living organisms on Earth, from plants to mammals, adhere to a daily or circadian rhythm. In humans, physiological states, intellectual abilities, and even mood undergo cyclical changes depending on the time of day. Researchers have demonstrated that these fluctuations are due to variations in hormone concentrations in the blood [7]. In recent years, significant advancements have been made in the field of chronobiology, the science of biorhythms, to uncover the mechanisms behind daily hormonal cycles. Scientists identified the "circadian center" as the suprachiasmatic nucleus in the brain and succeeded in isolating the Per, Time, and Clock genes, known as the "clock genes," which are essential to the rhythms of biological health [8].
In 1632, the English naturalist John Wren described the daily cycles of tissue fluids in the human body in his "Treatise on Herbs," using Aristotle's term "humor" (from Latin "humor" meaning liquid) to refer to these fluids. Wren noted that each "tide" of tissue fluid lasted six hours. The humoral cycle began at nine o'clock in the evening with the first humor of bile, known as "chole" (from Greek "chole" meaning bile), and continued until three in the morning. This was followed by the phase of black bile, referred to as "sadness" (from Greek "melas" meaning black, and "chole" meaning bile), then by phlegm (from Greek "phlegma" meaning mucus), and finally, blood. Of course, it's not possible to directly correlate these humors with the physiological fluids and tissue secretions known to modern medical science, which does not acknowledge any connection between these ancient concepts and physiology. However, the patterns of change in mood, intellectual capacity, and physical state described by Wren have a scientific basis in modern chronobiology, the study of the body's daily rhythms. This field's foundational concepts were developed by prominent German and American scientists, Jürgen Aschoff and Colin Pittendrig, who were even nominated for the Nobel Prize in the early 1980s [9].
During the circadian day (wakefulness), our physiology primarily focuses on recycling stored nutrients to provide energy for active daily activities. Conversely, during the circadian night, the body accumulates nutrients, and tissues are regenerated and repaired. These changes in metabolic rate are regulated by the endocrine system, i.e., hormones, showing parallels to Wren's humoral theory in the way the endocrine system controls circadian cycles. In the evening and during the dark period of the night, the "night hormone" melatonin is released into the blood from the pineal gland or epiphysis, the upper appendage of the brain. This remarkable substance is produced by the pineal gland only at night, and its presence in the blood is directly proportional to the duration of night. In some cases, insomnia in the elderly is linked to an insufficient release of melatonin by the pineal gland [10].
Material and Methods
Melatonin supplements are frequently used as sleep aids. Melatonin not only regulates sleep duration and phase changes but also causes a decrease in body temperature. It's well-documented that human sleep alternates between slow-wave and rapid eye movement (REM) phases. Slow-wave sleep is marked by low-frequency activity in the cerebral cortex, often described as "deep sleep," during which the brain is fully at rest. During REM sleep, the frequency of fluctuations in the brain's electrical activity increases, leading to dreams. This phase is similar to wakefulness and acts as a "springboard" for awakening. Throughout the night, slow-wave and REM phases alternate 4-5 times, with corresponding changes in melatonin concentration [11,12].
Beyond its role in regulating the sleep-wake cycle, melatonin has been identified and studied for many physiological functions, such as its antioxidant (twice as strong as alpha-tocopherol and five times stronger than glutathione), immunomodulatory, antihypertensive, anti-lipid, and anti-inflammatory effects. Recent research from 1997 to the present has significantly highlighted melatonin's anti-- cancer properties, drawing special attention from Japanese, Australian, Norwegian, and Chinese scientists to its potential use in cancer treatment in clinical settings. The exact mechanisms behind melatonin's anti-tumor effects remain unclear, and specific challenges complicate its in vivo studies. The anti-cancer effects of melatonin have been investigated in both spontaneous and induced tumors in animals. Ongoing research aims to explore the oncological effects of melatonin in humans with cancer. Additionally, the anti-tumor activity of melatonin has been established in cell cultures of various human tumor types.
Numerous studies have shown that melatonin in hibits the proliferation of malignant cells and triggers their apoptosis in tumor tissues, blocks the action of mutagens and clastogens, and suppresses the expression of oncogenes at the genetic level [1,5,7,17].
Research indicates that the process of carcinogenesis is significantly accelerated under constant light exposure, whereas it is inhibited in complete darkness. This observation has led to the development of the "dark therapy" method for cancer patients, which has moved beyond experimental oncology and is expected to become a standard part of clinical cancer treatment in the near future [7,8].
Additionally, it has been noted that during cancerous diseases, the concentration of melatonin in blood plasma decreases and pathological changes in the pineal gland are often observed. Interestingly, the extent of pineal gland damage correlates with the stage of the cancer disease rather than the tumor's location or origin [5,8,14].
Another intriguing finding is that melatonin levels in the blood plasma significantly increase during schizophrenia, a condition under which the incidence of cancer is notably low. Conversely, during depression and other forms of psychosis, when melatonin levels are substantially reduced, a higher incidence of cancer is recorded [9,11].
It has been established that tumors generally do not grow or grow less and are less aggressive at night, and they are less aggressive during the autumn and winter months compared to spring and summer. From a biorhythmological perspective, the plasma level of melatonin is higher during the dark hours and throughout the autumn-winter season [5,17].
Moreover, it is interesting to note that in individuals who are blind, the circadian and seasonal rhythms of melatonin synthesis and secretion are disrupted. From an oncological viewpoint, this is significant because statistical data suggest that blind women rarely develop breast cancer, and blind men are similarly less likely to develop prostate cancer [2,3,5,21].
Results and Discussion
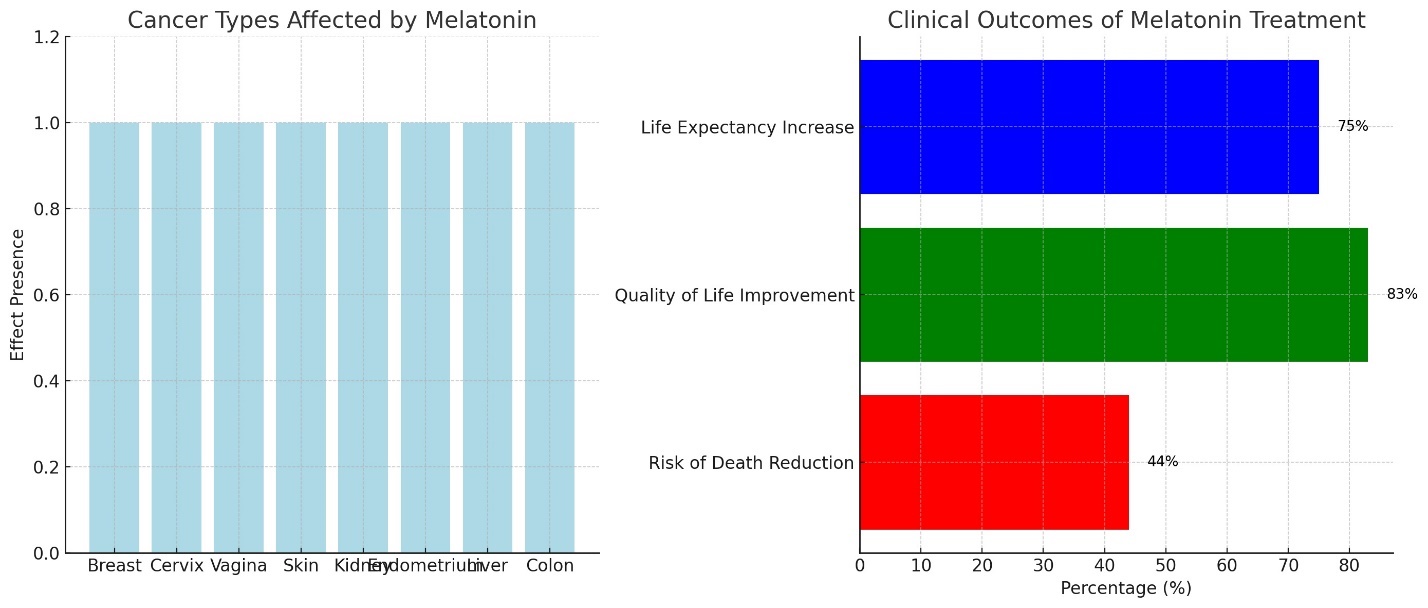
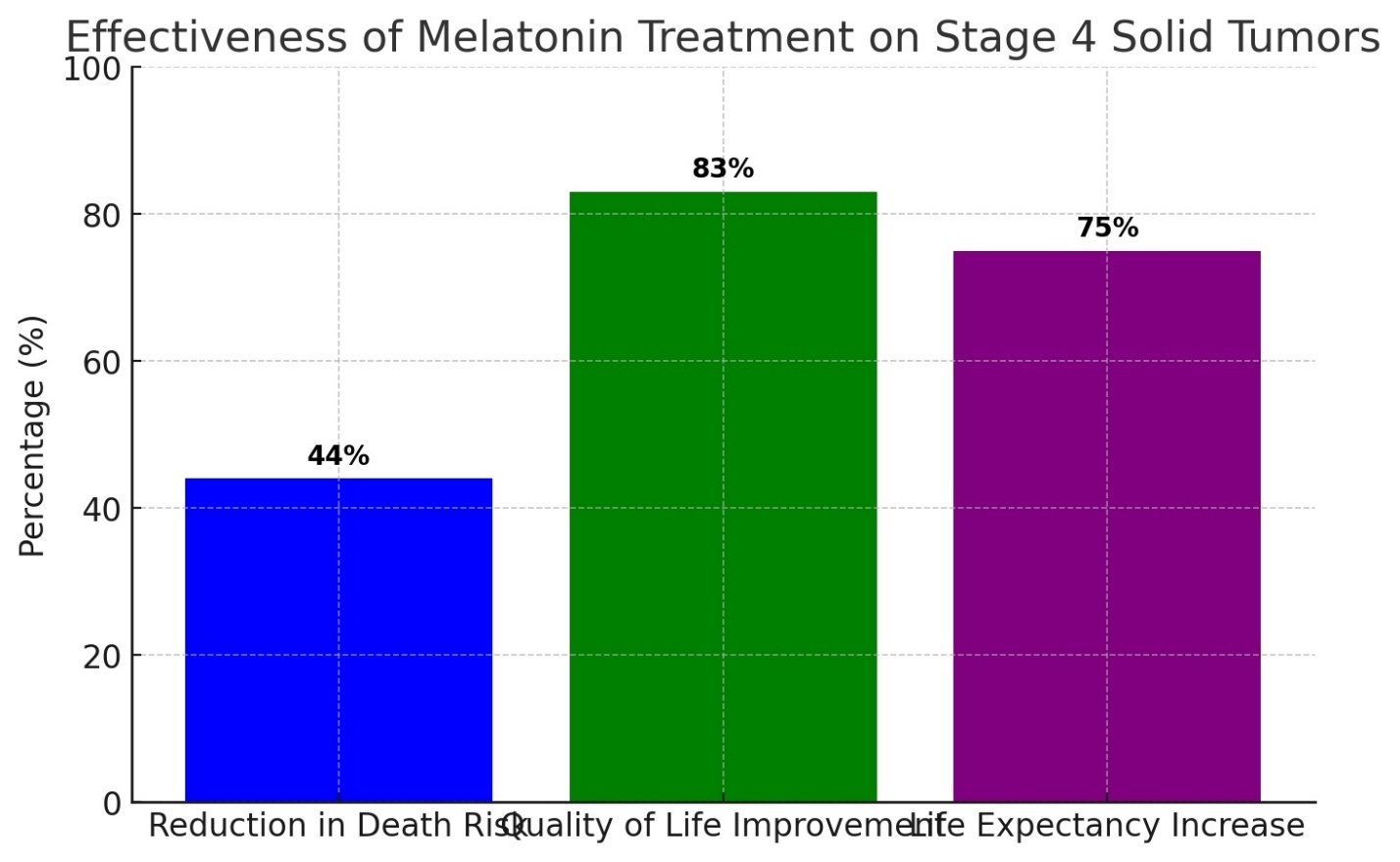
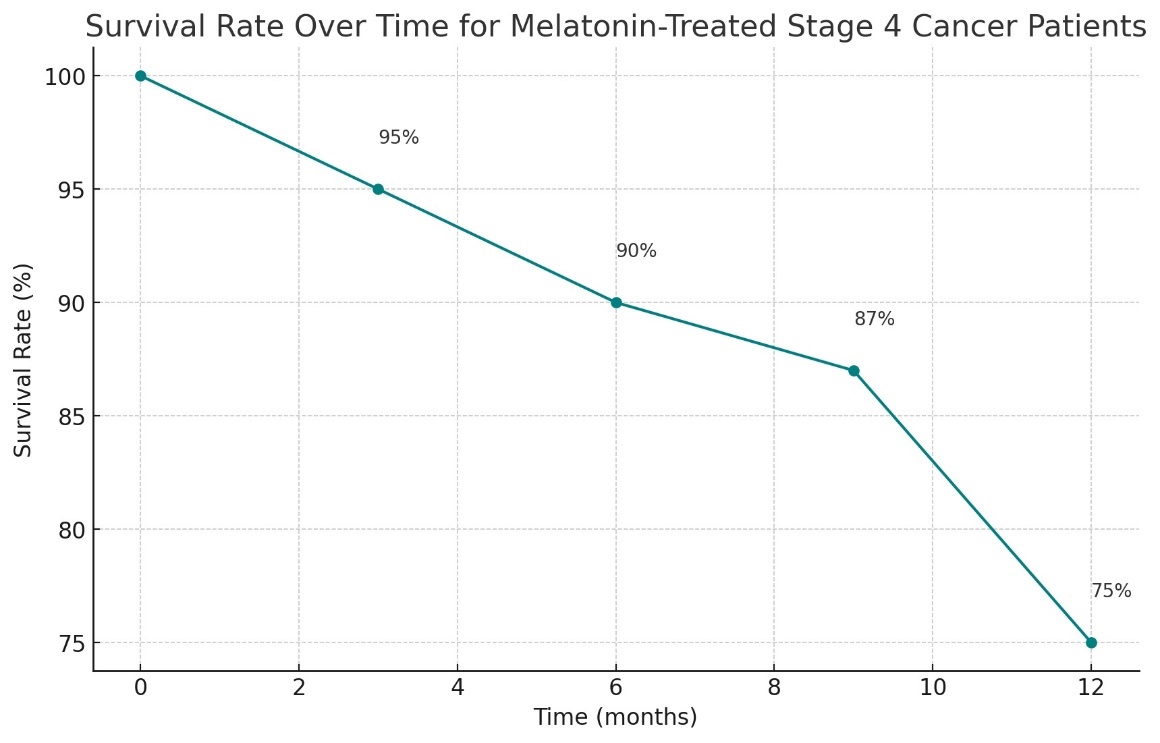
Research has shown that melatonin exerts a suppressive effect on malignant tumors of the breast, cervix, vagina, skin and subcutaneous tissues, kidneys, endometrium, liver, and colon. These findings align with the results of clinical observations. Clinical use of melatonin (400 mg or more per day in the form of rectal suppositories) was observed in patients with solid obesity. A study involving 643 patients with various solid tumors at stage 4, who were resistant to medical treatment and treated with melatonin monotherapy for one year, showed remarkable results. It was found that melatonin reduced the relative risk of death by 44%, improved the quality of life for 83% of the patients, and increased life expectancy by 75% compared to the statistical average. Notably, no side effects were detected in any patient during this period (Table 1, Figure 1) [19,25].
This visualization will offer insights into how effectively melatonin sustains or improves survival over a significant period. Melatonin, underscoring its potential in advanced cancer treatment. Here's the line graph illustrating the survival rate over time for patients with stage 4 solid tumors treated with melatonin: The graph shows a gradual decline in survival rate from 100% at the beginning of treatment down to 75% by the end of the year. The plotted points at each quarterly interval help visualize the survival trend across the year. This graph provides a clear depiction of how survival rates change over time in response to melatonin treatment, emphasizing its initial effectiveness and how it sustains over a year.
These findings underscore the significant role of the pineal gland, or epiphysis, in the upper appendage of the brain, in the development of cancerous diseases. The diminished function of the pineal gland under constant lighting—leading to suppressed melatonin secretion—stimulates carcinogenesis. Epidemiological observations have noted an increased risk of breast and colon cancer among night shift workers, findings that are consistent with rodent experiments. The administration of melatonin has been shown to suppress carcinogenesis in animals under both normal and constant light conditions. Thus, melatonin could be highly effective in cancer prevention, especially in northern regions where summers are marked by "white nights" and the long polar nights are illuminated by electric lights [13,15,16,17,26].
Unlike many other hormones, the effect of melatonin on cellular structures is influenced not only by its concentration in the blood and the extracellular environment but also by the initial state of the cell. This characteristic allows us to view melatonin as a universal endogenous adaptogen, which maintains homeostasis at a stable level and aids the body's adaptation to changing environmental conditions. Currently, many countries have approved melatonin formulations, registered either as medications or as biologically active supplements. There is now some experience with the successful use of these substances in various conditions, particularly in sleep disorders, gastric and duodenal ulcer disease, and hypertensive disorders. It's important to highlight the unique aspect of melatonin absorption, indicating that high-dose intake of 50 mg or more per day should be conducted under medical supervision, with periodic monitoring of blood melatonin levels and 24-hour urine melatonin sulfate. With the proper regimen and doses of melatonin, it's possible to fully regulate the body's disturbed biological rhythms, leading to the reversal of many pathologies and, in some cases, complete recovery [9,12,15,19,20,25].
The suppression of melatonin secretion in patients with malignant tumors initially drew our attention. Through extensive research, we discovered that dysregulation of melatonin secretion is one of the biochemical markers of cancer. Furthermore, we determined that the more advanced the cancer (regardless of its tissue origin: sarcoma, carcinoma, lymphoma, leukemia, malignant glioma), the more pronounced and simultaneous the decrease in melatonin secretion. This disruption in the body's biological rhythms leads to widespread metabolic desynchronization. This results in an excess of catabolic processes in cancer patients and promotes the progression of irreversibly destructive processes in the body as a whole system. By correcting these biorhythms, the process can often be reversed [11,14,17,24].
The evolution of the organic world occurs in the constantly changing conditions of the environment, with these changes often being cyclical. Consequently, the biophysical and biochemical processes in living organisms have developed a rhythmic nature. This implies that biorhythms serve as indicators of adaptation, through which we can assess the rhythm of biochemical process intensity, ultimately leading to the optimal distribution of energy over time. A system operating rhythmically utilizes energy as efficiently as possible and operates without loss. Living organisms possess a certain range of biorhythm variability, which is phylogenetically developed and genetically reinforced. Changes within these limits of biorhythm are permissible (physiological desynchronosis) and signify adaptive processes. However, significant distortion of the rhythm (inversion, dropout, etc.) indicates pathological desynchronization, suggesting that the biosystem can no longer regulate energy exchange, leading to self-destruction [18,21,22,23,27].
From this, we can deduce that desynchronosis causes disease, and disease itself causes further disruption, making the establishment of chronobiological methods in clinical medicine extremely important. Biorhythmological data can not only aid in effectively treating diseases but also in diagnosing and predicting them early. We identified a direct correlation between decreased melatonin secretion in the body and the progressive reduction of water channel receptors on the membranes of healthy cells. With age, a decrease in blood melatonin levels contributes to a deficiency of intracellular water in practically healthy individuals and the development of many age-related chronic diseases. In experimental models, we observed the genoprotective effects of liposomal melatonin (a molecular complex created in our laboratory) and its ability to activate genes encoding aquaporins (water channels). We also identified the pathophysiological changes that arise due to a deficiency of water channels in healthy cells of oncology patients and explored the potential outcomes and mechanisms of correction [2,3,5,7,16,24,25].
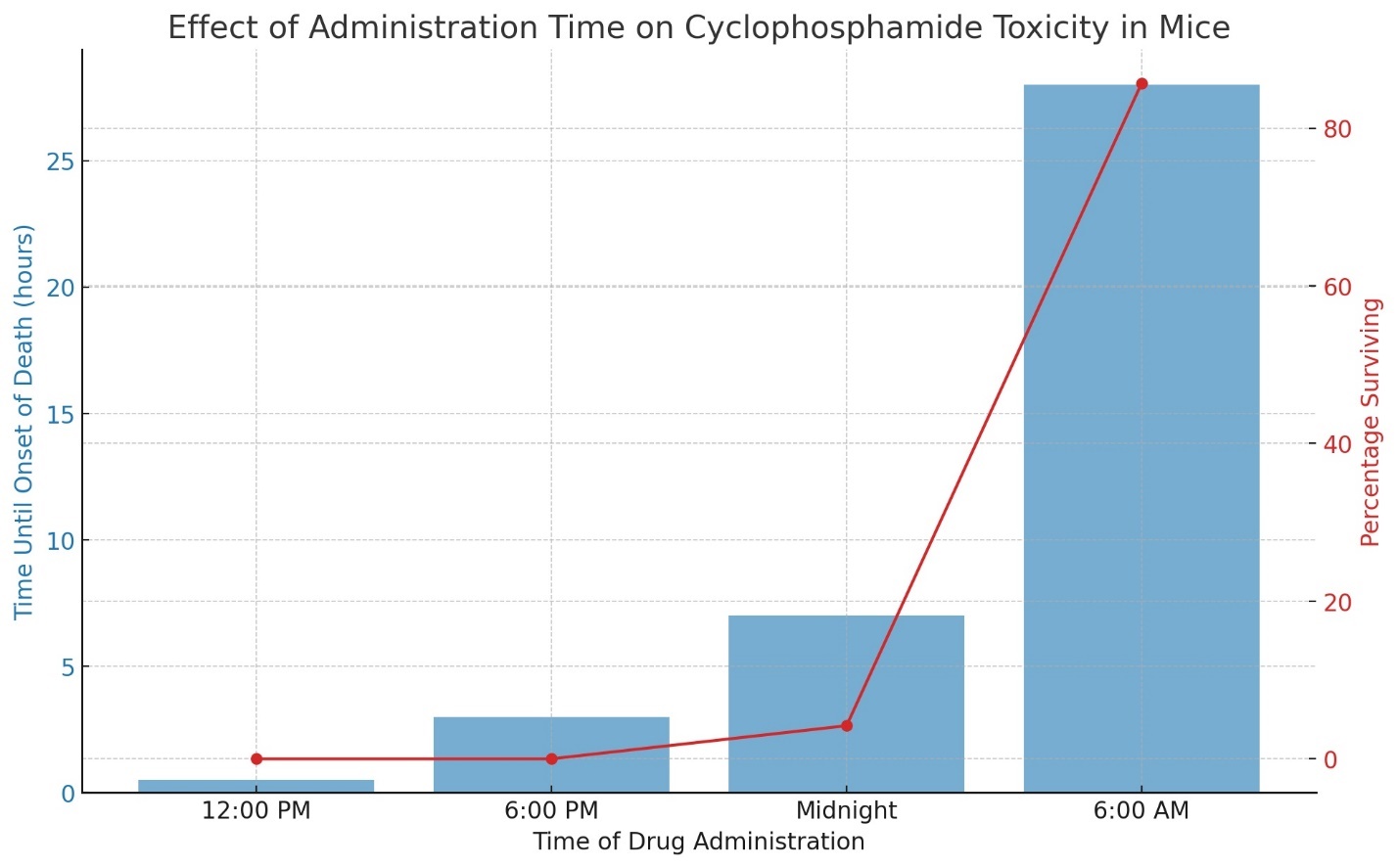
The essence of the chronobiological approach is prescribing medication at the time when its toxic effects on the body are minimized. Numerous experiments have demonstrated that the same dose of cyclophosphamide produces different outcomes at various times of the day and night. In one study, CHA-line mice were administered a dose of 900 mg of cyclophosphamide at 12:00 PM, resulting in all the animals dying within 30 minutes. When the injection was given at 6:00 PM, the animals began to die within 3 hours; at midnight, 7 hours post-injection, 95.8% of the mice were still alive before starting to die; and after receiving cyclophosphamide at 6:00 AM, all animals survived for 24 hours, with only 3 of the 21 mice dying at the 28th hour. This experiment revealed that CHA-line mice exhibit maximum resistance at 6:00 AM. Chronomedicine is poised to soon identify the optimal times for administering all medications, a goal that groups of French, Japanese, and Georgian scientists are actively pursuing [11,13,23].
Moden Scientific Research on Melatonin
In 2004, significant insights into how melatonin influences cancer were published, highlighting its potential in cancer prevention and treatment. Melatonin's protective roles against cancer initiation involve its antioxidant properties, which mitigate DNA damage caused by reactive oxygen and nitrogen species. This damage, if unrepaired, can lead to mutations and, eventually, cancer, particularly in the elderly due to the accumulation of such damage over time [17,19,26].
Melatonin has been shown to interfere with cancer promotion through multiple pathways. Notably, it limits the cellular uptake of linoleic acid (LA), a growth-promoting fatty acid. LA's conversion within cells triggers a cascade of events leading to cancer cell proliferation. This is significant given the high levels of n-6 fatty acids in modern diets and their association with increased cancer risks. Melatonin's presence in foods suggests its dietary intake could reduce cancer risks [14,27].
Moreover, melatonin has been found to inhibit telomerase activity, a key enzyme in cancer cell immortalization, thereby restraining the growth of certain cancer cells, such as MCF-7 human breast cancer xenografts. The reduction of telomerase activity highlights melatonin's potential to hinder cancer cell division and promote genetic stability [3,5,7,8,10].
Melatonin also exhibits antiestrogenic effects, which can limit the proliferation of hormone-sensitive breast cancers. This is mediated through the MT1 membrane receptor and possibly a nuclear binding site, underscoring melatonin's multifaceted role as an oncostatic agent. Although its impact through this pathway may be less pronounced than through LA uptake reduction, its effectiveness is well-documented across various studies [4,18,20,21].
Additionally, melatonin's ability to suppress endothelin-1 (ET-1) levels, a molecule implicated in cancer progression and angiogenesis, further emphasizes its potential in cancer therapy. ET-1 elevation in cancer patients aids tumor growth and metastasis by promoting blood vessel formation and preventing cancer cell apoptosis [1,2,7,19].
Prompted by these findings, the 2004 commentary advocated for melatonin's inclusion in clinical trials for cancer patients, citing preliminary but inconclusive evidence of its benefits in reducing cancer growth and enhancing patient wellbeing. Since then, the field has witnessed rapid advancements, with current data overwhelmingly supporting melatonin's efficacy in negatively affecting various cancer features. This bolsters the argument for clinical trials to explore melatonin as a therapeutic option against cancer [14,16,17,19].
This condensed summary reflects the breadth of research on melatonin's anticancer mechanisms, its preventive and therapeutic potential, and the ongoing call for clinical trials to validate its efficacy in cancer treatment. The selection of studies illustrates melatonin's impact on a diverse range of tumor types and underscores the importance of further investigation into its role in cancer therapy [23,25,27].
Cancer initiation involves genomic injury and instability, leading to an increased risk of mutation and cancer transformation. Various agents, including ionizing radiation, are significant contributors to cancer development, primarily affecting the genome. Ionizing radiation damages DNA through the absorption of high-energy photons or electrons, leading to mutations and tumor initiation. This damage is mediated by free radicals or reactive oxygen and nitrogen species, which are highly reactive and can alter DNA's biological functions. The direct effects of ionizing radiation include DNA mutations, while indirect effects result from molecular damage and physiological disruption [18,21,24].
Radioprotectors, both naturally occurring and synthetically produced, help mitigate ionizing radiation damage. These agents work by detoxifying free radicals, enhancing DNA stability, and promoting DNA repair, thus preventing mutations that could lead to cancer. Melatonin, identified early as a direct free radical scavenger, has proven to be an effective radioprotector and anti-cancer agent. It neutralizes hydroxyl radicals, which are responsible for a significant portion of genomic damage in cells exposed to ionizing radiation. Studies have shown melatonin's protective effects in vivo, including increased survival rates in irradiated mice and reduced genomic damage in bone marrow cells and blood lymphocytes [3,4,6,9,17].
Melatonin's radioprotective capabilities extend to reducing DNA damage and mutation frequency, underscoring its potential importance in cancer prevention. This is particularly relevant under conditions of prolonged lowdose radiation exposure, as seen after the Chernobyl accident or in the event of a radiological dirty bomb detonation. In the context of space travel, where high-energy space radiation can accelerate cellular aging and increase cancer risks, melatonin offers a physiological shield against molecular damage. Its application could also benefit space travelers by promoting sleep, stabilizing circadian rhythms, and maintaining bone and muscle health [4,5,9,17].
Given its endogenous production and lack of toxicity, melatonin is a promising candidate for mitigating the harmful effects of cosmic radiation, highlighting the need for strategies to reduce cancer incidence and other radiation-induced health issues in unique environments like space. Other DNA damaging agents and processes extend beyond ionizing radiation, involving both external and internal factors that contribute to genetic damage, potentially leading to cancer. Exogenous contributors include environmental pollutants, heavy metals, toxic drugs, and chemicals. Internally, endogenous processes can produce reactive oxygen species (ROS) beyond the antioxidant defense network's capacity, leading to damage. Misdirected electron flux in mitochondrial respiratory complexes and metabolic activities, particularly enzymatic processes, significantly contribute to ROS production. These reactive species are central to molecular damage, mutation, and cancer initiation, with substances like hydroxynonenal, a lipid peroxidation product, also having carcinogenic potential. Strategies that reduce ROS/RNS formation or enhance their neutralization can decrease cancer risk, highlighting the importance of mitochondria as major sites of free radical generation [3,4,7,11].
Melatonin, with its higher concentration in mitochondria, has been shown to effectively detoxify ROS, protecting mitochondria more efficiently than synthetically produced antioxidants. Classified as a mitochondria-targeted antioxidant, melatonin and its metabolites shield various macromolecules, including DNA, from damage, thus mitigating cancer precursor processes [17,19].
The role of transposable elements, especially the long interspersed element-1 (L1), in genomic stability is significant. Active L1s can cause de novo insertions per cell, leading to potential mutations through the production of proteins that facilitate retrotransposition, causing genomic DNA damage including double-strand breaks with mutagenic potential. Melatonin influences L1 expression, commonly upregulated in cancers, by reducing its mobility in cancer cells through the MT1 melatonin receptor interaction, highlighting the protective role of nighttime melatonin levels against DNA damage and the potential risks of reduced melatonin levels due to light pollution or aging [9,10,18].
DNA repair processes are crucial for reducing cancer initiation risk. The DNA damage response (DDR) system repairs various lesions, including oxidative modifications and strand breaks. When damage exceeds DDR capacity, cell death can prevent cancer if it occurs before cell replication. Although research is ongoing, evidence suggests melatonin may promote DNA repair, underscoring DDR's significance in genomic stability and its potential as a target for anticancer therapy. This comprehensive overview emphasizes the multifaceted roles of external and internal factors in DNA damage and the protective mechanisms against cancer, including the beneficial effects of melatonin and the importance of DNA repair processes [11,13,17,23].
Numerous studies confirm that melatonin slows the progression of various cancers in lab settings, reflecting decades of research and supporting epidemiological findings linking reduced melatonin levels with increased cancer risk. Despite consensus on melatonin’s anti-cancer effects and its lack of toxicity, its clinical use for cancer treatment remains limited. This underutilization is often attributed to melatonin's non-patentability and minimal financial incentives for its promotion. Research into melatonin's role in cancer is modestly funded and its significant laboratory findings have yet to be applied clinically [4,5,7,9,12,17,19].
Cancer cells often show increased reactive oxygen species (ROS) production due to metabolic abnormalities, contributing to cancer progression, resistance to chemotherapy, and tumor growth through mechanisms like angiogenesis. Some theories suggest that ROS may promote cancer cell proliferation by disrupting specific signaling pathways, thus enhancing cell growth and survival, including resistance to chemotherapy. However, the prevailing view supported by extensive research is that high ROS levels inhibit tumor growth and induce cancer cell death, particularly by sensitizing them to chemotherapy [3,5,9,21].
ROS-induced cancer cell death occurs through various pathways, including apoptosis triggered by cytochrome c release from mitochondria, autophagy, necroptosis, ferroptosis, and enhanced sensitivity to conventional chemotherapy. These mechanisms collectively underscore the potential of targeting ROS levels in cancer therapy [5,7,21].
The disruption of nocturnal melatonin levels and circadian rhythms by artificial light at night has been associated with higher cancer risks. This disruption complicates understanding whether increased cancer rates are due to melatonin suppression, circadian rhythm disturbances, or a combination of both. The influence of light pollution on cancer highlights the complex interplay between environmental factors, melatonin regulation, and circadian rhythm integrity in cancer development and progression [3,4,7].
Place and use of Melatonin in Traditional Chinese Medicine
Melatonin, a hormone primarily produced by the pineal gland in the brain, is well-known for its role in regulating sleep-wake cycles and its antioxidant properties. Beyond its physiological functions in the human body, melatonin also holds a significant place in traditional Chinese medicine (TCM), a holistic approach to health and disease that has been practiced for thousands of years. TCM encompasses a variety of practices, including herbal medicine, acupuncture, dietary therapy, and Tai Chi, and is rooted in the concept of balancing the body’s vital energy, or Qi, to maintain health and prevent illness [21,26].
In TCM, the concept of Yin and Yang is fundamental, representing opposite but complementary forces that need to be in balance for good health. Melatonin, with its calming and cooling properties, is often associated with Yin energy, which is considered to be nurturing, passive, and associated with nighttime and the moon. Its use in TCM is not directly named as "melatonin," but its effects are mirrored in the properties of certain herbs and practices aimed at enhancing sleep quality, reducing stress, and supporting the body’s natural healing processes [26].
Herbal Medicine: TCM utilizes a vast array of herbs that are believed to contain qualities similar to those of melatonin in terms of promoting sleep and alleviating stress. Herbs such as Suan Zao Ren (sour jujube seeds), Bai Zi Ren (biota seeds), and He Huan Pi (mimosa tree bark) are commonly prescribed to nourish the heart and calm the spirit, which in modern terms could be related to melatonin's sleep-enhancing and anxiolytic effects. These herbs are often used in complex formulas tailored to the individual's specific imbalance of Qi, rather than as isolated substances [3,21,26].
Dietary Therapy: In TCM, certain foods are believed to influence the balance of Yin and Yang in the body, and by extension, could be seen to affect the body’s production of melatonin or exhibit similar effects. Foods that are considered to have calming and cooling properties, such as lotus seeds, cherries, walnuts, and goji berries, are recommended for consumption in the evening to promote good sleep and to nourish Yin energy [26].
Acupuncture and Acupressure: These practices are central to TCM and involve stimulating specific points on the body to regulate the flow of Qi. Certain acupuncture points are believed to influence the body’s internal clock and could help in regulating sleep patterns and stress, possibly through mechanisms that might involve increasing the body’s natural production of melatonin or enhancing its efficiency [21,26,27].
Mind-Body Techniques: Tai Chi and Qi Gong are mind-body practices that combine movement, meditation, and breathing exercises to improve health and well-being. By reducing stress and promoting relaxation, these practices may help in regulating the body’s circadian rhythms and could potentially increase the natural production of melatonin [21].
While TCM does not directly reference melatonin in its classical texts, the principles of balancing Yin and Yang, nourishing the body with appropriate herbs and foods, and using acupuncture and mind-body techniques to reduce stress and improve sleep quality, all indirectly support the body’s natural melatonin function or mimic its effects. Modern research into the pharmacological actions of TCM herbs and practices increasingly suggests that many of the benefits attributed to these traditional remedies may involve mechanisms related to the modulation of melatonin levels or its receptor activity, bridging ancient wisdom with contemporary science [3,5,21,26].
Hypericum perforatum, commonly known as St. John's Wort, is a plant renowned for its medicinal properties, including its use as a treatment for depression, anxiety, and wound healing. Interestingly, it has also been identified as one of the plants with high concentrations of melatonin, a hormone known for regulating sleep-wake cycles and possessing antioxidant properties. The presence of melatonin in St. John's Wort adds to its therapeutic potential, offering insights into its effectiveness in treating sleep disorders and enhancing mental health [26].
Melatonin in St. John's Wort
St. John's Wort's rich melatonin content is significant for its implications in treating sleep-related issues and regulating circadian rhythms. Melatonin's role in the body extends beyond sleep regulation; it also acts as a powerful antioxidant, can support immune function, and has been studied for its potential in protecting against neurodegenerative diseases. The presence of melatonin in St. John's Wort complements its already well-documented effects on mood and neurological function, providing a multifaceted approach to mental and physical health [21,26]
St. John's Wort in Traditional Chinese Medicine (TCM)
In Traditional Chinese Medicine (TCM), St. John's Wort is not traditionally among the most commonly used herbs. This discrepancy arises from the different pharmacopeias and herbal traditions that have evolved over millennia in various cultures. TCM has its own set of herbs and formulations tailored to the theories of Qi (vital energy), Yin and Yang, and the Five Elements, which are foundational to its practice [23,26].
However, the principles underlying the use of St. John's Wort in Western herbalism can find parallels in TCM philosophy. For instance, TCM places a strong emphasis on the balance of emotional well-being, often treating mental health issues with herbs that "nourish the heart" and "calm the spirit." While St. John's Wort is not traditionally part of the TCM herbal lexicon, its effects on mood and sleep align with the TCM approach to treating similar conditions [7,21,26].
Herbs in TCM known for their calming and sleep- -promoting properties, such as Suan Zao Ren (Ziziphus jujuba var. spinosa) and Bai Zi Ren (Biota orientalis), are used to address issues of the heart and spirit, akin to how St. John's Wort is used in Western herbalism to treat depression and anxiety. The concept of treating the mind and body holistically is a shared theme between TCM and the use of St. John's Wort in herbal medicine [26].
Integration and Potential
The integration of St. John's Wort into TCM could be explored through the lens of its melatonin content and its known effects on mood and sleep. Practitioners of integrative medicine, who combine traditional practices with modern scientific knowledge, might find St. John's Wort a valuable addition to their pharmacopeia, especially for patients seeking natural remedies for depression, sleep disorders, and stress-related issues [21,26].
It's important to note that while St. John's Wort is beneficial for many, it can interact with a wide range of medications due to its effect on the cytochrome P450 enzyme system. This underscores the importance of consulting healthcare professionals before integrating it into one's regimen, particularly in the context of TCM, where herbal formulas are tailored to the individual's specific condition and constitution [21,26].
In conclusion, while St. John's Wort may not have a traditional place in TCM, its properties, particularly its rich melatonin content, align with the holistic approach to health and wellness that is central to TCM philosophy. Its potential integration into TCM practices highlights the evolving nature of traditional medicine in response to advances in phytochemical understanding and the global exchange of medicinal knowledge.
In conclusion, while melatonin as a concept is rooted in modern physiology, its roles and effects are paralleled in TCM through a holistic approach to health that emphasizes balance, natural rhythms, and the interconnectedness of body and mind. This ancient system of medicine offers a unique perspective on the use and significance of melatonin-like properties in promoting health and wellness [5,21,26].
St. John's Wort in Western Herbalism
St. John's Wort (Hypericum perforatum) is widely recognized for its antidepressant and anti-inflammatory properties, but it is less commonly known for its melatonin content. Melatonin is a hormone produced by the pineal gland in the brain, primarily responsible for regulating the body's circadian rhythms, including sleep patterns. While St. John's Wort is not typically highlighted for its melatonin content in comparison to its other active compounds, such as hypericin and hyperforin, recent studies suggest that it does contain melatonin and its precursors, which may contribute to its therapeutic effects [21,26].
Melatonin plays a crucial role in regulating sleep and is also involved in various biological functions, including immune system modulation and antioxidant activity. The presence of melatonin in St. John's Wort, though in smaller quantities compared to its primary active compounds, indicates potential benefits for sleep disorders and circadian rhythm adjustments. This discovery adds another layer to the complex pharmacological profile of St. John's Wort and may help explain some of its beneficial effects on mood and anxiety disorders, where sleep disturbance is often a comorbid condition [26].
Potential Benefits and Mechanisms
The melatonin found in St. John's Wort could potentially enhance its therapeutic effects, particularly for individuals experiencing sleep disturbances alongside depression or anxiety. Melatonin's well-documented sleep-regulating properties, combined with St. John's Wort's mood-enhancing effects, may offer a synergistic approach to treating these conditions. However, the exact mechanisms by which St. John's Wort influences melatonin levels in the body, and how this contributes to its overall effects, remain areas for further research [26].
Research and Considerations
Research on the melatonin content in St. John's Wort and its clinical significance is still emerging. Studies focusing on the extraction and quantification of melatonin in the plant, as well as its pharmacokinetic profile when ingested as part of St. John's Wort preparations, are necessary to fully understand its impact. Additionally, considering St. John's Wort's known interactions with various medications, understanding how its melatonin content interacts with other melatonin supplements or medications affecting the circadian rhythm is crucial for safe use.
In conclusion While St. John's Wort is not primarily known for its melatonin content, the presence of this hormone and its precursors in the plant may contribute to its multifaceted therapeutic profile. This aspect of St. John's Wort's pharmacology offers an intriguing avenue for research, potentially broadening its application in treating sleep-related disorders alongside its traditional use in managing depression and anxiety. As with all herbal supplements, consulting with healthcare professionals before starting any new treatment regimen is essential, especially given St. John's Wort's potential interactions with other medications [21,26].
Conclusion
The body of evidence points to melatonin's potential in cancer prevention and therapy, emphasizing the need for clinical translation of laboratory findings. Simultaneously, understanding the role of ROS in cancer provides a nuanced perspective on its dual functions in tumor biology, suggesting targeted approaches for cancer treatment. The implications of light pollution on melatonin and circadian rhythms further illustrate the broader environmental and physiological contexts affecting cancer risk, underscoring the importance of integrative research approaches to fully comprehend and effectively combat cancer.
The pharmaceutical formulation of melatonin is pivotal due to its short plasma half-life, variable oral absorption, low bioavailability, poor solubility, and stability, all compounded by extensive first-pass metabolism. Traditional oral dosage forms prove inadequate for melatonin delivery, prompting the development of diverse pharmaceutical formulations employing various approaches and administration routes to circumvent these limitations. Innovations include extended-release tablets using hydrophilic polymers, monolayered and three-layered tablets with polyvinylpyrrolidone and cellulose acetate, and the only licensed melatonin tablet in the UK, Circadin®, which exhibits a prolonged release profile unaffected by tablet division, Melatonin rectal suppositories are also very effective Melatonin SR 200 and 400 mg (from Zetpilnutrition and from Mitozen Scientific). Additionally, soft gel capsules have shown comparable pharmacokinetics to higher doses of melatonin powder, while formulations like melatonin-loaded porous starch and nanospheres have demonstrated significant improvements in pharmacokinetics and bioavailability. Intranasal administration presents a promising alternative, with nanoparticles enhancing melatonin's solubility and significantly lowering IC50 values in glioblastoma cells. Transdermal delivery, non-invasively targeting the site of action, and sublingual administration offering rapid absorption comparable to intravenous routes have also been explored. Complexation techniques have improved melatonin's solubility and bioavailability, underscoring the potential of innovative delivery systems in optimizing melatonin's therapeutic efficacy
Melatonin's role in cancer treatment and prevention has been extensively investigated, showcasing its anticancer effects across various cancers through apoptosis induction, immune system modulation, angiogenesis inhibition, and antimetastatic effects. Its combination with conventional therapies has reinforced therapeutic outcomes, enhancing chemotherapy efficiency and patient quality of life. Clinical studies advocate for melatonin's incorporation into cancer treatment regimens, highlighting its broad-spectrum anticancer effects and potential in cancer prevention. Despite its proven efficacy and low toxicity, the bioavailability and pharmacokinetic properties of exogenous melatonin warrant further investigation due to significant inter-individual variability. This variability emphasizes the need for continued research to fully understand melatonin's pharmacokinetics and optimize its clinical application in cancer prevention and treatment, supporting its role as a multifaceted agent in oncology [5,7,21,26,27].
In the form of conclusions
1. Experimental and clinical observations have established that melatonin exhibits multiple mechanisms of antitumor action, including antiproliferative, apoptosis-stimulating, endocrine and immune system modulating, and antiangiogenic activities.
2. Melatonin is produced in the upper part of the brain (epiphisis - pineal gland) during the dark period of the day and night and serves as the primary marker of circadian or daily biological rhythms.
3. Melatonin is unique because its receptors are found on all cells. It exists across a wide range of organisms, from the simplest to fungi, highlighting its significant biological importance.
4. Through its potent antioxidant activity, melatonin inhibits the initiation process of carcinogenesis. In experiments, melatonin reduced the mutagenic effect of the carcinogen N-methyl-nitrosourea by 1500 times compared to the control group.
5. Removing the pineal gland (epiphysectomy) in animals with cancer stimulates tumor growth
6. Exposing animals to 24-hour light for three months or more leads to the formation and development of various types of malignant tumors in 20-55% of laboratory animals due to suppressed melatonin secretion.
7. For animals with malignant tumors, maintaining a regime of constant darkness or administering pineal gland extract significantly slows, and sometimes completely halts, tumor progression.
8. Melatonin has been found to provide genoprotection to cancerous cells, activating enzymes involved in DNA repair under pathological conditions.
9. Tumors, especially hormone-dependent malignant ones, tend to grow less at night and are less aggressive in autumn and winter than in spring and summer.
10. Breast cancer is almost non-existent in blind women, and prostate cancer is similarly rare in blind men.
11. A significant increase in melatonin concentration, twenty times or more, is characteristic of severe mental illnesses such as schizophrenia. Melatonin is considered one of the molecular etiopathogenic factors of hallucinations in this pathology. The excess of melatonin also contributes to the rare occurrence of oncological diseases in patients with schizophrenia and their high resistance to infectious diseases.
12. In tumor tissues, melatonin inhibits the proliferation of malignant cells and activates their apoptosis, suppresses the action of mutagens and clastogens, and inhibits the expression of oncogenes at the genetic level.
13. During cancer, the concentration of melatonin in the blood plasma decreases, and pathological changes in the pineal gland are often observed. The extent of damage to the pineal gland correlates with the stage of the cancerous disease, not the tumor's location or origin.
14. Botanical experiments have shown that plant tumors disrupt the metabolism of heteroauxin, a substance whose content in plant biological fluids decreases. Heteroauxin is identical to human and animal melatonin, and its biosynthesis exhibits a circadian and seasonal rhythm.
15. Plasma levels of melatonin are moderately elevated in patients with Hodgkin's lymphoma. The age at which this disease emerges and its clinical manifestation directly correlate with the peak of endocrine activity of the pineal gland.
16. The judicious use of melatonin and other bioactive molecules, alone or in combination, in medical practice significantly improves the prognosis of oncological diseases and sometimes serves as universal preventive agents.
Informed Consent Statement
NA
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author Contributions
A. Tavartkiladze, G.Simonia, and L.Tavartkiladze conceived and designed the experiment; A. Tavartkiladze and D. Kasradze performed the experiments, analyzed the data, and wrote the manuscript; A. Tavartkiladze and L. Tavartkiladze contributed to data collection and manuscript revision; A. Tavartkiladze, G.Simonia and L.Tavartkiladze provided technical support and assisted with the experimental design. All authors contributed to manuscript revision and have read and approved the submitted version.
Acknowledgments
The authors are grateful to the Institute for Personalized Medicine for providing full-time access to genetics and molecular biology laboratories for a few weeks and Tbilisi State Medical University too.
Funding
This work was supported by the Institute for Personalized Medicine – PMI, Tbilisi, Georgia
Disclosure of Interest
The authors report no conflict of interest.
- Talib WH (2018) Melatonin and cancer hallmarks. Molecules, 23: 518.
- Li Y, Zhou Y (2017) Melatonin for the prevention and treatment of cancer. Oncotarget, 8: 39896-921.
- Slominski RM, et al. (2012) Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol, 351: 152-66.
- Hulvat MC (2020) Cancer incidence and trends. Surg. Clin, 100: 469-81.
- Talib WH et al. (2020) Plant-Derived Natural Products in Cancer Research. Molecules, 25: 5319.
- Cutando A et al. (2012) Role of melatonin in cancer treatment. Anticancer Res, 32: 2747-53.
- Luchetti F et al. (2010) Melatonin signaling and cell protection function. FASEB J, 24: 3603-24.
- Sanchez-Barcelo EJ et al. (2012) Melatonin uses in oncology. Expert Opin. Investig. Drugs, 21: 819-31.
- Talib WH (2020) A ketogenic diet combined with melatonin overcomes resistance in breast carcinoma. Nutrition, 72: 110659.
- Odeh LH et al. (2018) Synergistic effect of thymoquinone and melatonin against breast cancer. J. Cancer Res. Ther, 14: 324.
- Claustrat B, Leston J (2015) Melatonin: Physiological effects in humans. Neurochirurgie, 61: 77-84.
- Salehi B et al. (2019) Melatonin in Medicinal and Food Plants. Cells, 8: 681.
- Amaral FGD, Cipolla-Neto J (2018) A brief review about melatonin. Arch. Endocrinol. Metab, 62: 472-9.
- Slominski A et al. (2008) Melatonin in the skin. Trends Endocrinol. Metab, 19: 17-24.
- Reiter RJ (1991) Pineal melatonin: Cell biology of its synthesis. Endocr. Rev, 12: 151-80.
- Pourhanifeh MH et al. (2019) Potential use of melatonin in skin cancer treatment. J. Cell. Physiol, 234: 12142-8.
- Tan DX, Reiter RJ (2019) Mitochondria: The birth place of melatonin metabolism. Melatonin Res, 2: 44-66.
- Jockers R et al. (2016) Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol, 173: 2702-25.
- Liu J et al. (2016) MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol, 56: 361-83.
- Ng KY et al. (2017) Melatonin receptors: Distribution in mammalian brain. Brain Struct. Funct, 222: 2921-39.
- Arnao, M.B.; Hernández-Ruiz, J. Phytomelatonin: Sources, activities and world market. J. Funct. Foods 2018, 48, 37–42.
- Arnao MB, Hernández-Ruiz J (2014) Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci, 19: 789-97.
- Marta B et al. (2016) Exogenous melatonin improves antioxidant defense in cucumber seeds. Front. Plant Sci, 7: 575.
- Okazaki M, Ezura H (2009) Profiling of melatonin in tomato cultivar Micro-Tom. J. Pineal Res, 46: 338-43.
- B Arnao, M Hernández-Ruiz J (2018) The potential of phytomelatonin as a nutraceutical. Molecules, 23: 238.
- Reiter RJ, Rosales-Corral SA, Tan DX, Acuna-Castroviejo D, Qin L, et al. (2017) Melatonin, a Full Service Anti-- Cancer Agent: Inhibition of Initiation, Progression and Metastasis
- Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, Manchester LC (2007) Light-at-night, chronodisruption, melatonin suppression and cancer risk: A review. Critical Reviews in Oncogenesis, 13: 303-28.

Tables at a glance
Figures at a glance