Evolution of Axial Radiographic Damage in Patients with Psoriatic Arthritis: Relationship with Clinical Factors, Laboratory, and Ultrasound Enthesis Findings
Received Date: June 30, 2023 Accepted Date: July 31, 2023 Published Date: August 03, 2023
doi: 10.17303/jra.2023.2.101
Citation: L Gomez-Lechon Quiros, E Toledano Martinez, C Hidalgo Calleja, M Aguilar Gonzalez, ME Acosta de la Vega et al. (2023) Evolution of Axial Radiographic Damage in Patients with Psoriatic Arthritis: Relationship with Clinical Factors, Labora tory, and Ultrasound Enthesis Findings. J Rheumatol Arthritis 2: 1-14
Abstract
Background: Psoriatic arthritis (PsA) is a disease that affects multiple domains and can present itself in a very heterogeneous way. Peripheral involvement is frequently described, as well as its progression, which differs from that produced by other inflammatory arthropathies such as rheumatoid arthritis, that is why it has its specific progression indexes. There is less consensus when it comes to describing the axial involvement in these patients, at a practical level, it is frequently compared with involvement in ankylosing spondylitis and attempts have been made to use the same indexes to measure its radiographic progression. With this background, we propose our study with the objective of measuring radiographic progression in a sample of patients with PsA with axial involvement and associate it with clinical, analytical, ultrasound, and radiographic variables.
Objective: To measure the radiographic progression of a group of patients with psoriatic arthritis and axial involvement. We relate the findings to clinical, analytical, ultrasound, and radiographic factors.
Patients and Methods: This was a prospective longitudinal study in 45 patients diagnosed with psoriatic arthritis. Axial involvement was defined as inflammatory low back pain together with the presence of radiographic sacroilitis and/or syndesmophytes. Radiographic damage was measured by the PASRI method at the beginning and at four years. The presence of fracture was also evaluated in the radiographic analysis. At the beginning of the study, enthesis was assessed by the MASEI method. Radiographic progression was related to the following variables: age, gender, smoking habit, body mass index, peripheral involvement, dactylitis, modified MASES, PASI, NSAID (continuous vs on demand), biological DMARDs, ASDAS-CRP (mean), BASFI (baseline and differential), BASMI (baseline and at year 4) CRP (mean), and HLA-B27.
Results: Radiographic progression was greater in male gender (β: 1.40; p=0.04 IC95%: 0.07-2.73) and smoking (β: 1.63; p=0.01, IC95%: 0.40-2.97), R2: 0.23. It was also correlated with fracture (β: 1.84; p=0.004 IC95%:0.48-3.05), baseline PASRI (β: 0.42; p=0.001; IC95%: 0.04-0.18), R2: 0.37. and with baseline calcification of the enthesis (β: 0.36; p=0.01, IC95%: 0.03-0.28; R2:0.12). Twelve patients had no radiographic progression. These patients had a lower PASRI at baseline (5.25 + 2.63 vs 12.36 + 8.99; p = 0.004).
Conclusions: Male gender, smoking, baseline radiographic damage (including fracture), and enthesis calcification were associated with greater radiographic progression.
Keywords: Psoriatic Arthritis; Radiographic Progression; Ultrasound; Spondyloarthropathy
Introduction
Psoriatic arthritis (PsA) is an inflammatory disease associated with psoriasis that can affect the peripheral and axial joints [1]. Currently there are no unanimous classification criteria that define the axial manifestations of PsA, despite their importance since they can cause limited mobility and functionality with a worsening of quality of life [2-4]. For this reason, to date, the criteria for spondylitis have been used although the clinical and radiographic characteristics of both diseases may differ significantly. Thus, from a clinical viewpoint, there is greater cervical involvement in PsA, and radiographic damage does not always correlate with the intensity of spinal pain [5]. From a radiographic viewpoint, paravertebral ossification, the shape of the syndesmophyte (thick, marginal, paramarginal and asymmetrically distributed), and the asymmetric radiographic sacroilitis differs from that found in patients with spondylitis [6,7]. Furthermore, there seems to be less radiographic damage in patients with PsA and axial manifestation (PsAax) than in those diagnosed with spondylitis [8]. However, it is difficult to make this statement since to date there is not much evidence with direct studies that describe axial involvement in these two diseases. Due to these differences, we should not use factors that intervene in the radiographic progression of patients with spondylitis in patients with PsAax. However, there are currently no studies that assess these manifestations in patients with PsA. Since we have therapies that can intervene on axial damage, it would be relevant to know the factors that can be predictors of axial involvement. With this background, we propose our study with the objective of measuring radiographic progression in a sample of patients with PsAax and associate it with clinical, analytical, ultrasound, and radiographic variables.
Methods
A prospective longitudinal study was carried out in a PHC clinic in a tertiary hospital in the province of Salamanca (Spain) from November 2014 to December 2018.
A) Inclusion Criteria
Consecutive patients diagnosed with PsA according to CASPAR (ClASsification for Psoriatic ARthritis) criteria [9] who presented inflammatory axial pain (ASAS criteria) [10] of less than five years of evolution together with the presence of sacroilitis defined by the modified New York criteria [11] and/or one or more than one cervical or lumbar marginal or paramarginal syndesmophyte. The patients signed an informed consent. The study was carried out with the consent of the ethics committee of the Hospital Clínico Universitario de Salamanca (Ref 15/2013).
B) Baseline variables
Age, gender, duration of the disease (months), and smoking were separated into two categories: non-smoker (never smoked) and smoker (former smoker or current smoker). Body mass index (BMI) was used as a numerical value and categorized into normal (< 25) and overweight/obese (>25). Other variables include type of involvement (axial or mixed), dactylitis, enthesitis using the Maastricht Ankylosing Spondylitis Enthesitis Score modified (modified MASES). The MASES modified focuses on 15 entheseal sites (the bilateral first costochondral joints, seventh costochondral joints, posterior superior iliac spines, anterior superior iliac spines, iliac crests, proximal insertion of Achilles tendons, plantar fascia and the fifth lumbar spinous process). It is easy to calculate because the final score is given by the sum of the enthesis involved [12]. Non-steroid anti-inflammatory drug (NSAID) dosing (continuous vs “on demand”) [13], treatment with biological disease-modifying antirheumatic drug (bDMARDs), severity of psoriasis measured by Psoriasis Area Severity Index (PASI) [14], and HLA-B27 (CRP-SSOr Luminex) were also measured.
C) Variables to measure
Radiographic damage was measured using the Psoriatic Arthritis Spondylitis Radiology Index (PASRI) at the beginning and at four years (+ 1 month) to establish radiographic progression as the difference between both values. The PASRI, developed specifically for AxPsA, scores the sacroiliac (SI) joints individually from 0 to 4 using the New York scoring method, vertebral corners of the lumbar spine on both anteroposterior (AP) and lateral views from the lower thoracic T12 to upper sacral S1, and the anterior vertebral corners of the cervical (C) spine on lateral view from the lower C2 to the upper C6 (each corner scored from 0 to 3) and 1 point is added for every level of the facet joints (C2/C3, C3/C4, C4/C5, C5/C6) fused posteriorly[15].
In addition, the presence of fracture was assessed both at the dorsal and lumbar levels by means of Genant's semi-quantitative method where grade 1 (mild) fracture is a reduction in vertebral height of 25%, grade 2 (moderate) a reduction of 26–40%, and grade 3 (severe) a reduction of over 40% [16].
The enthesis damage was measured at the beginning of the study by the Madrid Sonographic Enthesis Index (MASEI) [17]. This method evaluates six entheses bilaterally (plantar fascia, Achilles tendon, quadriceps, proximal patellar, distal patellar, and triceps) measuring five elementary lesions (thickness, structure, erosion, calcification, bursitis, and power Doppler signal). The ultrasound system was a GE Logiq 5 Pro ultrasound system (General Electric Healthcare, Kyunnggi-do, Korea with a 7-12 MHz transducer probe).
The ultrasound and radiographic evaluation were performed with two trained observers (CM/LG) and by a radiologist (CO). All doubtful cases were discussed with CO making a final decision. The evaluation was performed without knowing the chronological order of the radiographs. The kappa index obtained between the two observers was 0.74 for the radiographic measurement and 0.70 for the ultrasound.
Measures of Activity, Functionality, and Mobility
Activity measurement was performed using the Ankylosing Spondylitis Disease Activity Score (ASDAS-CPR). ASDAS-CPR is obtained by the formula: 0.12 x back pain + 0.06 x duration of morning stiffness + 0.11 x patient global + 0.07 x peripheral pain / swelling + 0.58 x Ln (CRP+1) (18), ASDAS-CPR was calculated every year. Global means were used instead of individual values [19]. Bath Ankylosing Spondylitis Functional Index (BASFI) was used for functionality [20], and Bath Ankylosing Spondylitis Metrology Index (BASMI 3) was used for mobility [21]. These indexes were measured at the beginning and at the end of the study. The differential of these measures was established by subtracting the final result from the baseline result. CRP (mg/l) were measured at the beginning of the study and given the similar time intervals of laboratory determinations (four months). Global means were used instead of individual values [19].
Statistical analysis
Continuous variables were presented as mean (M) with standard deviation (SD) and categorical variables as numbers (N) and proportions (%). Comparisons between two groups were analysed using the χ² test for categorical variables, the independent sample T-test for continuous variables of normal distribution, and the Mann Whitney U test for continuous variables of abnormal distribution. The comparison between two qualitative variables was made with the chi square test. Bivariate correlations were performed between continuous variables using Spearman correlation coefficients (P-values were considered significant for values < 0.05). We performed three multiple linear regressions by steps between: the radiographic progression (dependent variable) and the clinical variables (gender and smoking) (independent variables), variables of radiographic damage (baseline PASRI and fracture) (independent variables), and the variables of baseline ultrasound damage (alteration of the structure and presence of calcification (independent variables). Due to the possible inference of the treatment on the radiographic progression, all models were adjusted for the use of bDMARDs. In this case, it is not adjusted by the use of NSAIDs, there is insufficient evidence to recommend continuous or on-demand use, since the potential benefit in the progression of radiological damage must be weighed against potential deleterious gastrointestinal and cardiovascular effects in each patient. It is necessary to clarify the concept of disease modification and validate more sensitive instruments for measuring structural damage, as well as longterm studies of NSAIDs to confirm their potential inhibitory action on bone fusion and to quantify the magnitude of cardiovascular risk based on the dose and the time of administration. This was an observational study, and an a priori calculation of the sample size was not performed to find differences between progressors and non-progressors. The statistical analysis was performed using SPSS version 20.
Results
General characteristics of the population
Forty-five patients met the inclusion criteria. There was no loss to follow-up in the cohort. The mean age of the patients was 46.62 years (SD: 5.50). The evolution time from the onset was 26.16 months (SD: 12.11). The baseline BASRI of the patients was 10.46 ± 8.40. There was a direct correlation between the duration of inflammatory axial pain and the baseline BASRI (R: 0.32; p < 0.03), but not with the age of the patients (R: 0.07; p = 0.6). The time of evolution (in months) of the disease was not related to gender (25.25 ± 11.98 vs 25.35 ± 12.82, p=0.64), smoking habit (26.75 ± 13 .03 vs 25.44 ± 11.84, p=0.64) or the presence of fracture (24.92 ± 9.01 vs 26.35 ± 13.43, p=0.72). The baseline characteristics of the patients are shown in table 1.
In bivariate correlations, the baseline PASRI (R = 0.49, P = 0.001) were also related to radiographic progression. Regarding ultrasound damage, total MASEI was related to radiographic progression (R = 0.35, p = 0.01). The analysis for different elementary lesions, we found a correlation with calcification (r = 0.36, p = 0.01) and structure (r = 0.32, p = 0.02) but not with thickening (r = 0.11, p = 0.43), bursitis (r = -0.27, p = 0.07), erosion (r = 0.14, p = 0.34), or power-Doppler (r = 0.02, p = 0.89 (table 3). There was no correlation with the rest of variables. There was also no correlation between the BASRI, BASFI (r = 0.22, p = 0.1), or BASMI (r = -0.11, p = 0.9) differentials (table 3).
The multiple linear regression using the radiographic progression as the dependent variable and the clinical variables, radiographic damage and variables of baseline ultrasound damage as independent variables are represented in table 4.
Discussion
We found that male gender, smoking, and previous axial structural damage influenced the radiographic progression of patients with PsAax. In addition to the axial manifestations of PsA, the presence of a morphometric fracture also intervened. The calcification of enthesis acted similarly.
We are unaware of another similar characteristics study in patients with PsA. We thus compared our results with those previously published in patients with spondylitis although the characteristics of both diseases may be different. Thus, the radiographic progression in our patients was less than that published in spondylitis. Effectively, radiographic progression in our patients was 0.62 units/year. This result is similar to a previous study [8]. Patients with spondylitis have been estimated to be between 1.5-2 units/year [22-26]. The difference between the two diseases could be even greater because we use the PASRI method instead of the mSASSS. This method incorporates damage to the cervical interapophyseals into the mSASSS thus increasing the scoring possibilities in patients with PsAax. More than 25% of our patients had no progression: While these data are similar to those found in spondylitis, it is difficult to establish parallels between both diseases due to the difference in follow-up time between the different series [22,27]. Despite these differences, many of the clinical features that accelerate radiographic progression coincided in both diseases. In most longitudinal studies in patients with spondylitis, male gender and smoking are associated with greater radiographic progression [25,26]. In other studies, the presence of the baseline syndesmophyte was the best predictor of radiographic progression [24].
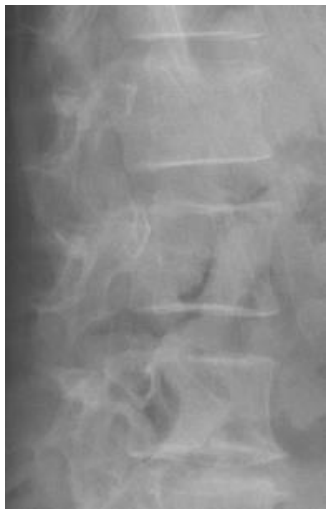
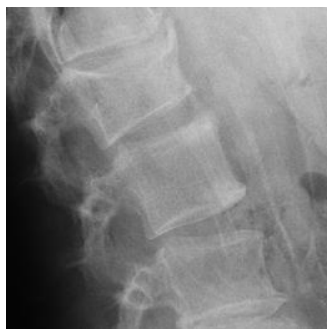
Morphometric fracture was related to radiographic progression. Although it may be surprising that more than 25% of our patients had a morphometric fracture, a previous study found 40% to have vertebral fractures with a higher fracture rate in patients with PsAax than in patients with spondylitis [28]. In patients with spondylitis, the presence of a fracture has been related to greater radiographic damage [29,30]. This fact could be secondary to two reasons: the first one, to the fact that axial immobility associated with radiographic damage would limit the patient's mobility and thereby influence bone fragility. However, it is necessary that other factors intervene in this association because the increase in radiographic progression may not have a significant influence on the mobility of the patient. The second reason, the presence of the syndesmophyte could change different biomechanical parameters that would decrease the resistance of the bone and with it an increase in the presence of fracture. In these cases, the sequence would begin in the syndemophyte and end in the vertebral fracture. We found an inverse relationship: radiographic damage was secondary to the morphometric fracture. Furthermore, individual analysis of the patients showed that some of the vertebrae that were fractured were those that had posterior radiographic progression (Figure 1 and Figure 2). We hypothesize that microtrauma would cause microfractures that would trigger the activation of progenitor cells and their transformation into osteoblasts mediated by bone morphogenetic protein (BMP); thus, the post-fracture remodelling process could participate in the formation of the syndesmophyte [31].
We found a correlation between the baseline sonographic damage of the enthesis and the subsequent radiographic progression. This significance was due to the alteration of the structure and the calcification of the enthesis in the univariate analysis and to the calcification in the multivariate analysis. These results partially coincide with those obtained by Polachek et al. who reported a cross-sectional study with a cohort of 223 patients. They correlated the damage in enthesis with the axial progression. The greatest association was obtained in the section of the MASEI that measures bone changes (tendon calcification and erosion) [32]. In another cross-sectional study in patients with spondylitis, structural damage in the spine (syndesmophyte) was related to the measured achillean entensophyte by ultrasound. This association is more frequent in men and is called the “bone-forming phenotype” [33]. Due to the design of our study, we cannot affirm that the ultrasound findings of peripheral enthesis precede axial damage; this aspect, if confirmed, would have a great clinical significance since the ultrasound examination of these entheses in patients with low back pain of inflammatory characteristics could be useful when it comes to discriminating which patients would be more likely to develop a more aggressive spinal phenotype. We cannot know if there was a correlation between the progression of damage in enthesis and bone damage because we did not perform the ultrasound measurement at the final visit. We did not find a correlation between the progression of radiographic damage and the decrease in mobility or functionality. This result can be justified by the duration of the disease. Previous studies have found that the greatest correlation between mobility limitation and radiographic damage occurred in patients with more than 10 years of disease evolution [4].
One of the limitations of the study was the small size of the sample, which is a consequence of the exhaustive inclusion criteria in the recruitment of patients. In PsAax, there is a dissociation between the symptoms and the axial radiographic damage that is proportional to the time of disease evolution [4,34]. This fact causes the presence of different stages in the radiographic symptoms-damage relationship, the stage where there is a greater correlation between the symptoms and the radiographic damage is the one with the shortest evolution time of the disease. Thus, made us include patients in a homogeneous way within a relatively short time interval with a reduced evolution of the disease. This ensures that all patients were within the same stage and that the clinical manifestation and radiographic damage were the most consistent in this stage. This is why the progression of damage in our study did not depend on the time of evolution of the axial manifestations prior to inclusion.
The limited number of patients subtracts statistical power from the study. In our patients, we did not obtain a correlation between the ASDAS-CRP mean and progression, although we did find a trend that could possibly have had statistical significance in the case of a larger population. On the other hand, the small group of patients included led us to categorize the smoking habit into two categories (smoker/non-smoker) instead of having categorized it into three (former smoker/smoker/non-smoker). The same situation occurred with BMI.
Another limitation of the study stems from the lack of unanimity to define PsAax [35-37]. We included patients con inflammatory low back pain clinical and radiographic manifestations. Although the criteria that define have a lower specificity and sensitivity in PsAax than in spondylitis [38], however, at the present time there are no more accurate criteria available.
In addition, we use radiographic damage because it is possibly the best way to define patients with PsAax in clinical practice due to the limited accessibility that exists in most centres for routine nuclear magnetic resonance imaging. This definition has been used in clinical trials for the treatment of this manifestation of the disease [39].
As a conclusion, we can say that although the radiographic axial progression in patients with PsA is less than in patients with spondylitis and that it does not influence mobility or functionality in a short space of time, there can be factors that influence a worse quality of life in future. With these background and with the possibility of using treatments that inhibit radiographic structural damage, the results of our study place the extent of the baseline radiographic damage as a predictor of greater structural progression to thus define “the therapeutic window” in patients with minimal structural damage; therefore, those patients with “rapid progression” (men, smokers, or those with ultrasound damage in enthesis) should be closely monitored and identified as early candidates for the use of these therapies [40].
Author contributions
All the authors have participated in the bibliographic search and preparation of the following manuscript.
L. Gómez-Lechón Quirós: bibliographic search, case acquisition and writing - original draft, E. Toledano: review, writing and ultrasound imaging assessment, C. Hidalgo Calleja: bibliographic search, review and editing, M. Aguilar González bibliographic reseach and editing, M.E. Acosta de la Vega: bibliographic search, Ruben Queiró Silva supervision, review, C.A. Montilla Morales: editing, supervision, review.
Acknowledgements
Dr. M. Aguilar González (Hospital de Manises, Valencia, Spain) for content editing
E. Aristoy Gómez-Lechón (Hospital Universitario La Fe, Valencia, Spain) for English editing.
Ethical Statement
The patients involved agree to include their clinical data for the publication of the manuscript. All mandatory laboratory health and safety procedures have been complied with in the course of conducting any experimental work reported in the manuscript.
We declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We know no conflicts of interest associated with this publication, and there has been no financial support for this work that could have influenced its outcome. As Corresponding Author, I confirm that the manuscript has been read and approved for submission by all the named authors with subsequent modifications. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. The publication is approved following the guidelines of the ethical committee of the University Hospital of Salamanca (CEIM Hospital Universitario de Salamanca. (Ref: 15/2011).
- Chandran V, Barrett J, Schentag CT, Farewell VT, Gladman DD (2009) Axial psoriatic arthritis: update on a longterm prospective study. The Journal of Rheumatology 36: 2744-50.
- Hanly JG, Mitchell MJ, Barnes DC, MacMillan L (1994) Early recognition of sacroiliitis by magnetic resonance imaging and single photon emission computed tomography. The Journal of Rheumatology 21: 2088-95.
- Baraliakos X, Coates LC, Braun J (2015) The involvement of the spine in psoriatic arthritis. Clinical and experimental rheumatology 33: S31-5.
- Chandran V, Barrett J, Schentag CT, Farewell VT, Gladman DD (2009) Axial psoriatic arthritis: update on a longterm prospective study. J Rheumatol 36: 2744-50.
- Salvarani C, Macchioni P, Cremonesi T, Mantovani W, Battistel B, Rossi F et al. (1992) The cervical spine in patients with psoriatic arthritis: a clinical, radiological and immunogenetic study. Ann Rheum Dis 51: 73-7.
- McEwen C, DiTata D, Lingg C, Porini A, Good A, Rankin T (1971) Ankylosing spondylitis and spondylitis accompanying ulcerative colitis, regional enteritis, psoriasis and Reiter's disease. A comparative study. Arthritis and rheumatism 14: 291-318.
- Helliwell PS, Hickling P, Wright V (1998) Do the radiological changes of classic ankylosing spondylitis differ from the changes found in the spondylitis associated with inflammatory bowel disease, psoriasis, and reactive arthritis? Ann Rheum Dis 57: 135-40.
- Jadon DR, Sengupta R, Nightingale A, Lindsay M, Korendowych E, Robinson G et al. (2017) Axial Disease in Psoriatic Arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis 76: 701-7.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54: 2665-73.
- Sieper J, van der Heijde D, Landewe R, Brandt J, Burgos-Vagas R, Collantes-Estevez E et al. (2009) New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Ann Rheum Dis 68: 784-8.
- van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27: 361-8.
- Heuft‐Dorenbosch L, Spoorenberg A,van Tubergen A, Landewe R, van der Tempel H, Mielants H et al. (2003) Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 62: 127-32.
- Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Marker-Hermann E, Zeidler H et al. (2012) Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 71: 1616-22.
- Fredriksson T, Pettersson U (1978) Severe psoriasis: oral therapy with a new retinoid. Dermatologica 157: 238-44.
- Lubrano E, Marchesoni A, Olivieri I, D'Angelo S, Spadaro A, Parsons WJ et al. (2009) Psoriatic arthritis spondylitis radiology index: a modified index for radiologic assessment of axial involvement in psoriatic arthritis. J Rheumatol 36: 1006-11.
- Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res Off J Am Soc Bone Miner Res 8: 1137-48.
- de Miguel E, Munoz-Fernandez S, Castillo C, CoboIbanez T, Martin-Mola E (2011) Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis 70: 434-9.
- Lukas C,Landewé R, Sieper J et al. (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68: 18-24.
- Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ 300: 230-5.
- Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P et al. (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21: 2281-5.
- van der Heijde D, Landewé R, Feldtkeller E (2008) Proposal of a linear definition of the Bath Ankylosing Spondylitis Metrology Index (BASMI) and comparison with the 2-step and 10-step definitions. Ann Rheum Dis 67: 489-93.
- Ramiro S, Stolwijk C, van Tubergen A, van der Heijde D, Dougados M, van den Bosch F et al. (2015) Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow-up of the OASIS study. Ann Rheum Dis 74: 52-9.
- Poddubnyy D, Haibel H, Listing J, Marker-Hermann E, Zeidler H, Braun J et al. (2012) Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Ann Rheum Dis 64: 1388-98.
- van Tubergen A, Ramiro S, van der Heijde D, Dougados M, Mielants H, Landewe R (2012) Development of new syndesmophytes and bridges in ankylosing spondylitis and their predictors: a longitudinal study. Ann Rheum Dis 71: 518-23.
- Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F et al. (2014) Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis 73: 1455-61.
- Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J (2016) High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis 75: 2114-8.
- Montilla C, Diaz-Alvarez A, Calero-Paniagua I, Collantes-Estevez E, Font P, Almodovar R et al. (2014) Ankylosing spondylitis without axial progression: analysis of associated factors. J Rheumatol 41: 2409-12.
- van der Weijden MA, van der Horst-Bruinsma IE, van Denderen JC, Dijkmans BA, Heymans MW, Lems WF (2012) High frequency of vertebral fractures in early spondylarthropathies. Osteoporosis int 23: 1683-90.
- Ghozlani I, Ghazi M, Nouijai A, Mounach A, Rezqi A, Achemlal L et al. (2009) Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone 44: 772-6.
- Kang KY, Kim IJ, Jung SM, Kwok SK, Ju JH, Park KS et al. (2014) Incidence and predictors of morphometric vertebral fractures in patients with ankylosing spondylitis. Arthritis Res Ther 16: R124.
- Lories RJ, Luyten FP, de Vlam K (2009) Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther 11: 221.
- Polachek A, Cook R, Chandran V, Gladman DD, Eder L (2017) The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthritis Res Ther 19: 189.
- Aydin SZ, Can M, Alibaz-Oner F, Keser G, Kurum E, Inal V et al. (2016) A relationship between spinal new bone formation in ankylosing spondylitis and the sonographically determined Achilles tendon enthesophytes. Rheumatol int 36: 397-404.
- Queiro R, Belzunegui J, Gonzalez C, De DJ, Sarasqueta C, Torre JC et al. (2002) Clinically asymptomatic axial disease in psoriatic spondyloarthropathy. A retrospective study. Clin Rheumatol 21: 10-3.
- Lubrano E, Parsons WJ, Marchesoni A, Olivieri I, D’Angelo S,Cauli A et al. (2015) The definition and measurement of axial psoriatic arthritis. J Rheumatol Suppl 93: 40-2.
- Lubrano E, Spadaro A (2012) Axial psoriatic arthritis: an intriguing clinical entity or a subset of an intriguing disease? Clin Rheumatol 31: 1027-32.
- Fernandez-Sueiro JL (2009) The challenge and need of defining axial psoriatic arthritis. J Rheumatol 36: 2633-4.
- Yap KS, Ye JY, Li S, Gladman DD, Chandran V (2018) Back pain in psoriatic arthritis: defining prevalence, characteristics and performance of inflammatory back pain criteria in psoriatic arthritis. Ann Rheum Dis 77: 1573-7.
- Mease PJ, Helliwell PS, Gladman DD, Poddubnyy D, Baraliakos X, Chakravarty SD et al. (2021) Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol.
- Braun J, Baraliakos X, Deodhar A, Poddubnyy D, Emery P, Delicha EM, Talloczy Z, Porter B (2018) Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology (Oxford).

Tables at a glance
Figures at a glance