Minimum CQA targets |
Results |
SVF with >70% viability of nucleated cells |
90.7% |
SVF with >10,000 live nucleated cells per ml of concentrated lipoaspirate enzymatically processed |
58,000 live nucleated cells per ml of concentrated lipoaspirate enzymatically processed |
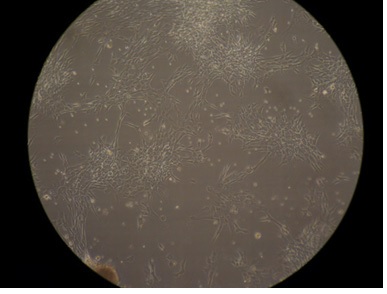
ASC plastic adherence |
ASC plastic adherence: confirmed |
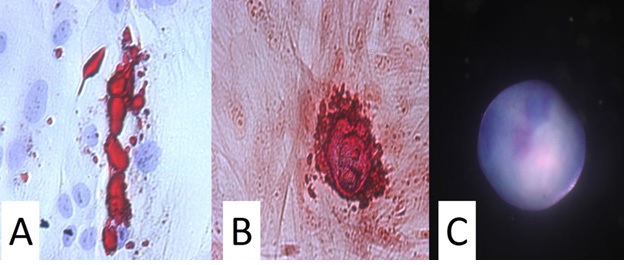
ASC tri-lineage differentiation
- Adipogenic
- Chondrogenic
- Osteogenic
|
ASC tri-lineage differentiation
- Adipogenic: confirmed
- Chondrogenic: confirmed
- Osteogenic: confirmed
|
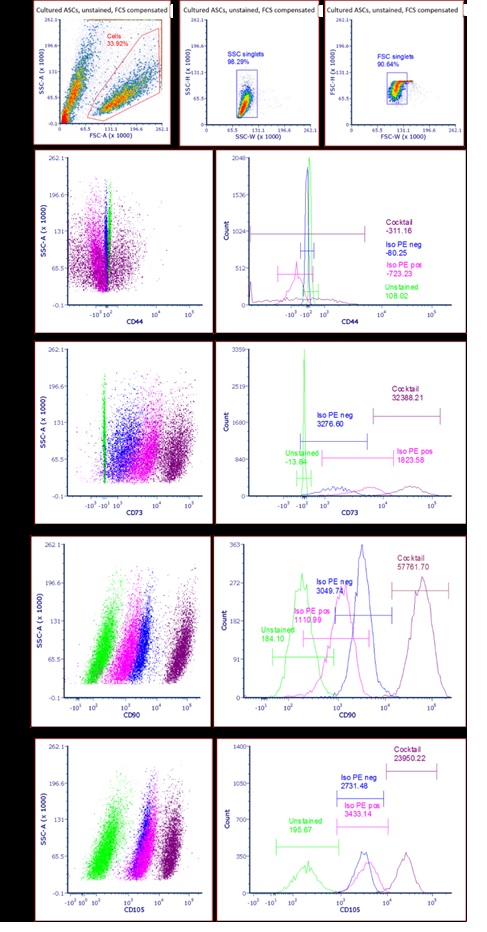
ASC Immunophenotype
- +: CD90, CD73,CD105,CD44
- -: CD11b,CD19,CD34,CD45,HLA-DR
|
ASC Immunophenotype
- +: CD90, CD73: confirmed
- +CD105: confirmed
- +CD44: confirmed
- -: CD11b,CD19,CD34,CD45,HLA-DR:
all confirmed |
Endotoxin level: <0.01 EU/ml |
Confirmed: <0.004 EU/ml |