Preliminary Study on the Expression of PI3K/ AKT/mTOR Signal Pathway During the Chondrogenesis of hMSCs
Received Date: September 27, 2021 Accepted Date: October 27, 2021Published Date: October 29, 2021
doi: 10.17303/JSCR.2021.3.102
Citation: Zhang Li-jun (2021) Preliminary Study on the Expression of PI3K/ AKT/mTOR Signal Pathway During the Chondrogenesis of hMSCs. J Stem Cell Rep. 3: 1-11.
Abstract
It is one of the effective ways to treat cartilage defects by inducing MSCs to differentiate into chondrocytes to construct cartilage engineering. Cytokines can regulate cell differentiation by mediating signal pathways. In order to investigate whether PI3K/AKT/mTOR signaling pathway is involved in the process of TGF-β3 induced the chondrogenesis of hMSCs, hMSCs were divided into Blank control group, TGF-β3 group and TGF-β3+LY29004group and then were induced into cartilage pellets respectively. At day 4,7,14,21, the pellets were collected and detected the relative expression of PI3K , AKT and mTOR by qRT-PCR and Western blotting. qPCR results showed that the mRNA expression of PI3K, AKT and mTOR was higher than that of the control group after induction by TGF-β3. Otherwise, for adding PI3K inhibitor LY29004 group, the expression of PI3K, AKT and mTOR was significantly down-regulated. The WB results showed that TGF-β3 can also up-regulate the expression of PI3K, AKT and mTOR protein, and the expression was significantly down-regulated after the inhibitor LY29004 was added. The above results indicate that TGF-β3 can initiate the expression of key genes in the PI3K/AKT/mTOR signaling pathway during the directional differentiation of hMSCs induced by TGF-β3.
Keywords: TGF-β3; hMSCs; Chondrogenic Differentiation; PI3K/AKT/mTOR Signaling Pathway
Introduction
Once the articular cartilage is damaged, it is difficult to repair because articular cartilage lacks blood vessels and nerves [1]. The traditional microfractures, cartilage transplantation and other treatment effects are not ideal [2]. It is reported that micro-fracture is helpful in short term in cartilage degeneration, but cartilage degeneration is expected irrespective of the size of the lesion more than 5 years after the surgery [3]. The disadvantages of using autograft include donor site morbidity and limited available graft volume while the main problem with allograft is graft host reaction and availability of allograft [4-5]. Autologous chondrocyte transplantation has achieved good results in clinical applications.however,the disadvantages of this procedure include that it is a complex procedure and it requires longer recovery time for maturation of new tissue [6].Therefore, tissue engineering technology to regenerate articular cartilage has become the research focus [7]. Mesenchymal stem cells have the ability of self-proliferation and multidirectional differentiation. They can differentiate into chondrocytes under certain conditions, so it is one of the effective ways to treat cartilage defects by inducing MSCs to differentiate into chondrocytes to construct cartilage engineering [8,9].
In the process of inducing mesenchymal stem cells to differentiate into cartilage, cytokines such as TGF-β,BMPs,FGFs play an important role, but the mechanism of this effect is still unclear [10-12]. Studies have shown that cytokines can bind to corresponding receptors on the cell membrane of stem cells, and then initiate intracellular signaling pathways including Wnt/β-catenin, MAPK/ERK and Notch, activate downstream signaling molecules, regulate the expression of transcription factor genes, and ultimately regulate the chondrogenic differentiation ability of hMSCs [13-15]. PI3K/AKT/mTOR signaling pathway plays an important role in regulating cell functions such as cell proliferation, differentiation, apoptosis and cell cycle pregression [16]. Studies have shown that the PI3K/AKT/mTOR signaling pathway promotes the survival of chondrocytes and the synthesis of extracellular matrix [17]. However, the activation of this signaling pathway has not been reported in the process of inducing the differentiation of hMSCs into chondrocytes. In this paper, the expression of key genes in the PI3K/AKT/mTOR signaling pathway were investigated during the process of differentiation of hMSCs into chondrocytes, which is expected to lay a foundation for the in-depth study of the mechanism of PI3K/AKT/mTOR signaling pathway in mediating chondrocyte differentiation.
Materials and Methods
hMSCs Culture
hMSCs (provided by Cyagen Bioscience Inc.,Guangzhou, China) were cultured in basal medium (Gibco, Grand Island, SÁ) containing 10% fetal bovine serum (Gibco), 1% 5mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco), with the condition of 37°C and 5% CO2 . Cells were passaged according to the conventional methods and the 6th passage was used for experiments.
Cartilage pellet method induces chondrogenic differentiation of hMSCs
Treatment groups: Cells were divided into control group, TGF-β3 group, and TGF-β3+LY29004 (PI3K inhibitor, ) group. The control medium is the complete medium for adult bone marrow mesenchymal stem cells. The TGF-β3 group medium is the control medium containing 10 ng/mL TGF-β3. TGFβ3+LY29004 group medium is TGF-β3 group medium containing 0.2 ㎛ of LY29004
Differentiation induction method: hMSCs at passage 6 were treated with trypsin. After treatment ,cells were centrifuged at 2000 rpm for 5 min and resuspended in differentiation medium at a density of 5.0×105 cells/ml, then cultured in a CO2 incubator (37°C, 5% CO2).After 24 h, the tube was flicked at bottom to make the cartilage pellet detach from the bottom of the tube and suspend in the culture medium[18].
RNA extraction and quantitative RT-PCR analysis
At days 4, 7, 11, 14, and 21 of chondrogenic differentiation, cell pellets from each group were harvested for quantitative RT-PCR (qRT-PCR) analysis. Total RNA was extracted using Trizol kit (Qiagen,Hilden, Germany). Isolated RNA was reverse using Reverse Transcription Kit (Invitrogen, Carlsbad, SÁ). Quantitative PCR analysis was performed using SYBR Green Supermix (Bio-Rad, Hercules, CA), and primers are listed in Table 1. Reaction conditions: 50°C 2min; 95°C 2min; 95°C 15s, 60°C 32s, a total of 40 cycles. Relative expression of each target mRNA was measured by 2-ΔΔCt method, With 18sRNA as the internal reference gene.
Protein extraction and western blot
At days 4, 7, 11, 14, and 21 of chondrogenic differentiation cell pellets from each group were harvested for western blot analysis. Cells were lysed with RIPA buffer (Pierce, Rockford, IL) containing protease inhibitor cocktail (Sigma, St. Louis, MO.). The supernatant was collected after centrifugation at 12000g for 30min at 4 °C. Protein concentrations were detected using a BCA Protein Assay kit (Thermo Scientific). Approximately 10-30 μg protein/well was separated by SDS-PAGE and transferred to an polyvinylidene difluoride (PVDF) (Millipore Corp., Bedford, MA). Membranes were blocked in 5% skim milk in TBSTween 20 (0.03%) and incubated with different primary antibodies against PI3K , AKT,mTOR (Abcam, Cambridge, UK)and GAPDH ((Abcam, Cambridge, UK) as reference. Blots were developed using chemiluminescence with Amersham ECL reagents (GE, Buckinghamshire, UK). The signal intensity of each target protein was quantified using densitometry and normalized to that of GAPDH.
Statistics
Mean values of all quantitative assays were calculated from at least three replicate samples. Statistical analysis was performed using SPSS-17.0 software.Parametric data are presented as means ± SD and compared using one-way ANOVA or a Student’s t-test Statistical significance was set at a P value of <
Results
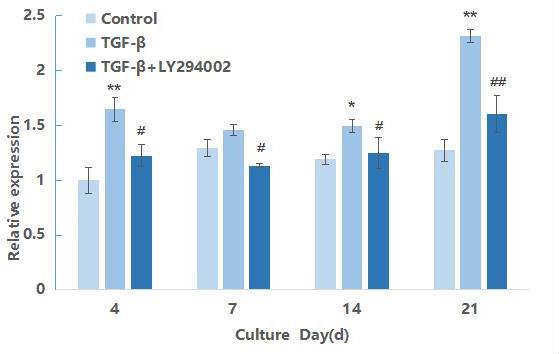
PI3K mRNA expression during differentiation of hMSCs induced by TGF-β3
PI3K is the primary gene in the PI3K/AKT/mTOR signaling pathway. Therefore, the expression of PI3K gene at the mRNA level was first detected in the process of inducing the differentiation of hMSCs into chondrocytes, The results are shown in Figure 1. At the four time points of induction, the relative expression of TGF-β3 group was significantly higher than that of the blank control group (P<0.05), and the expression reached the highest on the 14th day of induction.At the 21st day after induction, the the expression declined. For the TGF-β3+LY29004 group, the relative expression of PI3K was significantly higher than that of the blank control group, but compared with the TGF-β3 group, the expression was significantly down-regulated with signal pathway inhibitor added, and At the 14th and 21st days of induction, the mRNA expression level was significantly lower than that of the group without inhibitors (P<0.01). These results indicated that TGF-β3 can activate the transcription of PI3K during the differentiation of hMSCs into chondrocytes.,
AKT mRNA expression during differentiation of hMSCs induced by TGF-β3
AKT is a downstream gene of PI3K in the PI3K/AKT/mTOR signaling pathway. So, on the basis of detecting the expression of PI3K gene, the expression at the level of AKT mRNA is further detected. The results are shown in Figure 2. As shown in the figure, at the four time points of induction, the relative expression level of TGF-β3 group was significantly higher than that of the blank control group (P<0.05).But, the expression of AKT mRNA did not increase significantly along with the extension of induction time and only at the 21st day the expression of AKT has an obvious trend of up-regulation.It may be that AKT is the downstream gene and the activation would be prolonged after PI3K gene activation during the differentiation of hMSCs into chondrocytes. For the inhibitor LY29004 group, the expression level was significantly lower than that of the non-addition group, and at 21 days of induction, the mRNA expression level was extremely significantly lower than that of the non-inhibitor group (P<0.01). These results indicated that after PI3K is inhibited the expression of AKT mRNA was further down-regulated and delayed.
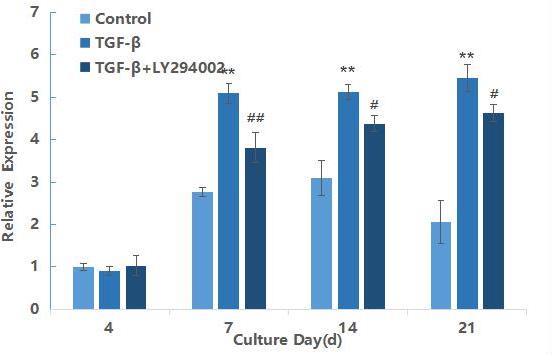
mTOR mRNA expression during differentiation of hMSCs induced by TGF-β3
mTOR is a downstream gene of AKT in the PI3K/AKT/mTOR signaling pathway, and its expression results are shown in Figure 3. After 7 days of induction, the relative expression of TGF-β3 group was extremely significantly higher than that of the blank control group (P<0.01), and the expression level reached the maximum at 21 days of induction. This result further indicated as the downstream gene mTOR was also activated with the prolonged induction time. In comparison between the TGF-β3 and TGF-β3+LY29004 groups, the expression of mTOR in the TGF-β3+LY29004 group was significantly down-regulated (P<0.05). Furthermore,these results indicated that the PI3K/AKT/mTOR signaling pathway can be activated and expressed during the process of TGF-β3 differentiation into chondrocytes.
As far as AKT (Figure 4C) is concerned, during the induction process, the relative expression in TGF-β3 group was significantly higher than that of the blank control group (P<0.05), and as the induction time prolonged, the protein expression increased. However,because as the downstream gene, the expression level reached the highest at day 21.In comparison between TGF-β3 and TGF-β3+LY29004 groups, after 14 days induction, the expression level in TGF-β3+LY29004 group was significantly lower than that of TGF-β3 group (P<0.01).
Protein expression of PI3K/AKT/mTOR signaling pathway during differentiation of hMSCs induced by TGF-β3
On the basis of the detection results of the expression of mRNA level of the PI3K/AKT/mTOR signaling pathway, Western blotting was used to detect the protein levels expression of the above three genes. The IOD analysis results are shown in Figure 4. The results showed that after 14 days of induction, the relative expression of PI3K (Figure 4B) in TGF-β3 group was significantly higher than that of blank control group (P<0.01) and at day 14 the expression level reached the highest. For the TGFβ3+LY29004 group, after 14 days of induction the relative expression of PI3K was significantly lower than that of the TGF-β3 group (P<0.01) under the action of the inhibitorLY29004, which was as the same as the expression of its mRNA.
For mTOR protein (Figure 4D), after 14 days of induction, the protein expression in TGF-β3 group was significantly higher than that of the control group. Moreover, the protein expression in the TGF-β3+LY29004 group was significantly lower than that in the TGF-β3 group (P<0.01). On the other hand, with the extension of the induction time, the increase of protein expression was as the same as the expression of mRNA, and there was also the case of delayed expression because of downstream gene. The above-mentioned protein expression results of PI3K, AKT and mTOR in the process of inducing hMSCs into chondrocytes also indicated that TGF-β3 can activate this signal pathwa
Discussion
Cartilage damage is an important pathological manifestation of many orthopedic diseases, such as osteoarthritis [1]. Because cartilage tissue lacks the distribution of blood vessels, nerves and lymphatics, it is generally difficult to repair articular cartilage after damage, which has become one of the problems in clinical treatment. In recent years, with the development of tissue engineering cartilage repair, mesenchymal stem cells have shown good results in repairing cartilage defects and are expected to become candidates for cartilage regeneration [19-21
The growth and differentiation of cartilage is a complex biological process. It is regulated by a variety of cytokines, and multi-factor interaction between cells [1]. Cytokines are important components for inducing MSCs into cartilage differentiation and culture in vitro. Most previous studies have confirmed that TGF-β3(transforming growth factor β3) is a potential ideal factor for inducing MSCs into cartilage differentiation [22-25].
TGF-β can bind to specific type II receptors, recruit corresponding type I receptors, initiate a series of cascade reactions that lead to the phosphorylation of its specific receptor Smads (R-Smads), and then transfer to the nucleus to regulate the expression of the cartilage-related gene, thus the chondrogenic differentiation ability of MSCs can be regulated [22]. Roelen et al. [26] further confirmed that TGF-β signaling plays an important role in the Smad3 pathway, because TGF-β can inhibit the adipogenic differentiation.of MSCs [27]. TGF-β1 also can activate the ERK/JNK signaling pathway and stimulate MSCs to differentiate into cartilage and cartilage formation [28-29]. Recent studies have shown that TGF-β1 activates the ERK/JNK pathway to increase the role of vitamin D in inducing the proliferation, migration and chondrogenic differentiation of bone marrow mesenchymal stem cells [30]. Our previous study showed that TGF-β3 could differentiation of MSCs into chondrocyte [18].
The PI3K/AKT/mTOR pathway controls important normal cellular processes including cell survival, proliferation, regulation of cell cycle, angiogenesis, and metabolism [17]. phosphatidylinositol 3-kinase (PI3K) is a downstream effector of G-coupled protein receptors and tyrosine kinase receptors. PI3K is consisting of a p110 catalytic and a p85 regulatory subunit. When the growth factors bind to the membrane receptor, it activates P85 to recruit P110 to activate it near the cell membrane, which in turn catalyzes the production of phosphoinositide diphosphate (PIP2) on the inner surface of the membrane to phosphoinositide triphosphate (PI
As a second messenger, PI3P activates Akt and phosphatidylinositol-dependent kinase 1.Akt plays a very important role in regulating cell size, growth, proliferation, survival, and sugar metabolism. When Akt is activated, it phosphorylates TSC2, which relieves the inhibition of Rheb (Ras homolog enriched in brain) by TSC1/2, and Rheb activates mTOR (mammalian target of rapamycin). mTOR is an important serine/threonine protein kinase,which activates downstream target proteins to regulate cell growth, metabolism, and protein synthesis[31]. Recent studies have shown the PI3K-AKT signaling pathway was related to proliferation,differentiation and apoptosis of chondrocyte. Feng, et al. found that Artesunate also inhibited chondrocyte proliferation and accelerates cell apoptosis and autophagy via suppression of the PI3K/AKT/mTOR signaling pathway [32]. Zhang reported that over-expression of FN1 contributes to fracture healing by activation of the TGF-β/PI3K/Akt signaling pathway [33]. The family with sequence similarity 3A (FAM3A),a mitochondrial protein that plays an important role for cellular adaptation to stress and cell survival, activated PI3K/Akt/mTOR pathway in IL-1β-treated chondrocytes, and blockade of PI3K/Akt/mTOR pathway with specific inhibitors, wortmannin and LY294002, diminished FAM3A effect on IL-1βinduced chondrocyte apoptosis, hence demonstrating that FAM3A attenuates IL-1β-induced chondrocyte apoptosis through activating the pro-survival PI3K/Akt/mTOR pathway[34].Chen et al. reported that addition of specific PI3K inhibitor LY294002 to culture of condyle chondrocytes could inhibit chondrocyte proliferation relative to the control and increased chondrocyte hypertrophy and type II collagenase expression .But, there were few reports about could differentiation of MSCs into chondrocyte [35].
Conclusion
In this study, we induced the chondrogenesis of hMSCs with TGF-β3, uninduced group as control group and adding specific PI3K inhibitor LY294002 group as comparison group.At days 4, 7, 14, and 21, the cells were collected and the expressions of PI3K, AKT and mTOR were detected by qRT-PCR and Western blot. For PI3K gene expression, the qRTPCR results showed that the relative expression of TGF-β3 group was significantly higher than that of the blank control group (P<0.05), and the expression reached the highest on the 14th day of induction. Otherwise, the relative expression of PI3K was significantly down-regulated with signal pathway inhibitor added (P<0.01),especially at 14th and 21st day. The WB analysis result was as the same as the expression of mRNA.With regard to downstream gene AKT and mTOR, qRT-PCR results showed that the expression of AKT and mTOR in TGF-β3 group increased compared with the control group (P<0.05),but it was not positively correlated with induction time. For TGF-β3+LY29004 group, the expression of AKT and mTOR was down-regulated by addition of LY294002 , and at 21st day the mRNA expression level of Akt was extremely significantly decreased vs TGF-β3 group (P<0.01).However, there was a significantly betweenTGF-β3+LY29004 group andTGF-β3 group for mTOR mRNA expression(P<0.05) .WB results showed that the protein expression of Akt was almost as the same as mRNA exprssion. For mTOR, the protein expression in TGF-β3+LY29004 group was significantly decreased compared with TGF-β3 group only after 21 days induction. These results indicated that as the downstream genes of PI3K, the expression of Akt and mTOR was further down-regulated and delayed after PI3K was inhibited. In this paper, the results suggested that TGF-β3 induced differentiation of hMSCs into chondroblasts maybe by acting PI3K/AKT/mTOR signaling pathway. However, there still need more researches to verify the role of PI3K/AKT/mTOR signaling pathway during the differentiation of hMSCs into chondroblasts.
Acknowledgments Science and Technology Planning Project
This research was supported in part by the Natural Science Foundation of Guangdong Province (Grant No.2018A030313048) and the Science and Technology Planning Project of Shenzhen science and Technology Innovation Committee (Grant No. JCYJ20170306144035589).
- Jafri MA, Kalamegam G, Abbas M (2020) Deciphering the Association of Cytokines, Chemokines, and Growth Factors in Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Using an ex vivo Osteochondral Culture System[J]. Front Cell Dev Biol 7: 380.
- Ketansinh S. Shanmugasundaram S, Kim SJ (2021) Articular cartilage repair & joint preservation: A review of the current status of biological approach.Journal of Clinical Orthopaedics and Trauma 21 : 101602.
- Goyal D, Keyhani S, Lee EH (2013) Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy 29: 1579-88.
- Bentley G, Greer III RB (1971) Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature 230: 385-8.
- You Q, Liu Y, Duan XJ (2017) Articular cartilage defect repair with particulated juvenile cartilage allograft: existing problems and prospects. Zhongguo Zuzhi Gongcheng Yanjiu 21: 5565-70.
- Efe T, Theisen C, Fuchs-Winkelmann S (2012) Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results.Knee Surg Sports Traumatol Arthrosc 20: 1915-22,
- Arshi A, Petrigliano FA,Williams RJ (2020) Stem Cell Treatment for Knee Articular Cartilage Defects and Osteoarthritis[J]. Curr Rev Musculoskelet Med 13: 20-7.
- Julien F, Dan B, Richard B (2016) Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways,safety and efficacy – a review[J]. BMC Musculoskeletal Disorders 17: 230-40.
- Mamidi MK, Das AK, Zakaria ZR (2016) Mesenchymal stromal cells for cartilage repair in osteoarthritis[J]. Osteoarthritis and Cartilage 24: 1307-6.
- Huang Z, He G, Huang Y (2017) Deferoxamine synergizes with transforming growth factor-beta signaling in chondrogenesis[J]. Genet Mol Biol, 2017, 40(3): 698-70.
- Zhou N, Li Q, Lin X (2016) BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells[J]. Cell Tissue Res 366: 101-11
- Danisovic L, Varqa I, Polak S (2012) Growth factors and chondrogenic differentiation of mesenchymal stem cells[J]. Tissue cell 44: 69-73.
- Tao Y, Zhou X, Liang C (2015) TGF-beta3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling[J] . Growth Factors 33: 326-36.
- Jiang X, Huang B, Yang H (2017) TGF-β1 is involved in Vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway[J]. Cell Physiol Biochem 42: 2230-41.
- Brady K, Dickinson SC, Guillot PV (2014) Human fetal and adult bone marrow-derived mesenchymal stem cells use different signaling pathways for the initiation of chondrogenesis[J]. Stem Cells Dev 23: 541-54.
- Dongl X, Quan Z, Yun G (2020) PDK1 is important lipid kinase for RANKL-induced osteoclast formation and function via the regulation of the Akt-GSK3β-NFATc1 signaling cascade[J]. J Cell Biochem 121: 4542-57.
- Chang L, Zhao D, Liu HB (2015) Activation of sonic hedgehog signalingenhances cell migration and invasion by induction of matrix metalloproteinase-2 and-9 via the phosphoinositide-3 kinase/AKT signaling pathway in glioblastoma[J].Mol Med Rep 12: 6702-10.
- Li QQ, Zhang LJ, Huang ZL (2018) Effects of recombinant human interferon β1a on the chondrogenic differentiation of human bone marrow mesenchymal stem cell[J]. Chin J Biotech 34: 1−9.
- Liu Y, Ma Y, Zhang J (2019) Exosomes: A Novel Therapeutic Agent for Cartilage and Bone Tissue Regeneration[J]. Dose Response 17: 1559325819892702.
- Iijima H, Isho T, Kuroki H (2018) Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation[J]. NPJ Regen Med 3: 15.
- Garza JR, Campbell RE, Tjoumakaris EP (2020) Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial[J]. Am J Sports Med 48: 588-98.
- Blanchi VJ, Weber JF, Waldman SD (2017) Formation of Hyaline Cartilage Tissue by Passaged Human Osteoarthritic Chondrocytes[J].Tissue Eng Part A 23: 156-65.
- Wang T, Nimkingratana P, Smith CA (2019) Enhanced chondrogenesis from human embryonic stem cells[J]. Stem Cell Res 39: 101497.
- Chijimatsu R, Kobayashi M, Ebina K (2018) Impact of dexamethasone concentration on cartilage tissue formation from human synovial derived stem cells in vitro[J]. Cytotechnology 70: 819-29.
- Mahboudi H, Soleimani M, Enderami SE (2018) Enhanced chondrogenesis differentiation of human induced pluripotent stemcells by MicroRNA-140 and transforming growth factor beta 3 (TGFβ3)[J]. Biologicals 52: 30-6.
- Roelen BAJ, Dijke PT (2003) Controlling mesenchymal stem cell differentiation by TGF β family members[J]. J Ortho Sci 8: 740-8.
- Kim EK, Choi E (2015) Compromised MAPK signaling in human diseases: an update[J]. Arch Toxicol 89: 867-82.
- Yu B, Yu D, Cao L (2011) Simulated microgravity using a rotary cell culture system promotes chondrogenesis of human adipose-derived mesenchymal stem cells via the p38 MAPK pathway [J]. Biochem and Biophys Res Commun 414: 412-1
- Tao Y, Zhou X, Liang C (2015) TGF-beta3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling [J]. Growth Factors 33: 326-36.
- Jiang X, Huang B, Yang H (2017) TGF-β1 is involved in Vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway [J]. Cell Physiol Biochem 42: 2230-41.
- Jason SL, Wei Cui (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signaling in Pluripotencyand cell fate determination[J]. Development. 143: 3050-60.
- Feng FB, Qiu HY (2018) Effects of Artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis[J]. Biomedicine & Pharmacotherapy 12: 1209-20.
- Zhang HL, Chen X, Xue P (2021) FN1 promotes chondrocyte differentiation and collagen production via TGF-β/PI3K/Akt pathway in mice with femoral fracture[J]. Gene 769: 145253
- Yan S, Jiang CQ, Li H (2019) FAM3A protects chondrocytes against interleukin- 1β-induced apoptosis through regulating PI3K/Akt/mTOR pathway[J]. Biochemical and Biophysical Research Communications, 516: 209-14
- Chen LL, Huang M, Tan JY (2013) PI3K/AKT Pathway Involvement in the Osteogenic Effects of Osteoclast Culture Supernatants on Preosteoblast Cells[J]. Tissue Engineering : Part A 19: 2226-32.

.jpg)
.jpg)
.jpg)
.jpg)
Tables at a glance
Figures at a glance