Stem Cells and Their Therapeutic Applications for Human Diseases: A Review
Received Date: December 23, 2021 Accepted Date: January 23, 2022 Published Date: January 25, 2022
doi: 10.17303/jscr.2022.4.101
Citation: Roozbeh Almasi (2022) Stem Cells and Their Therapeutic Applications for Human Diseases: A Review. J Stem Cell Rep. 4: 1-18.
Abstract
Despite being widely used in the treatment of various diseases, stem cells still need further study due to their many advantages and disadvantages. The possibility of attaching these cells to damaged organs has encouraged many researchers to understand their use. Stem cells are highly divisible cells that can differentiate into different types of other cells, and some, such as nerve cells, may lose the ability to divide. Today, researchers are using methods to use stem cells for chronic conditions such as heart disease, neurological diseases, diabetes, and more.
The safety and efficacy of using stem cells derived from blood or bone marrow to regenerate hematopoietic stem cells have been demonstrated. Regular controlled trials and standards for such promising treatments are complex and require the widespread use of these cells in clinical trials.
The most important ethical problem in the use of these cells is related to the origin of the cell. Due to the destruction of embryonic stem cell embryos (ESCs), its moral issues are more challenging than the use of adult stem cells. Also, embryo production for the sole purpose of treatment, instrumental use of humans and disrespect for human embryos, fertility for abortion, commercialization of embryo production, cloning, etc. are among the moral problems of using these cells. Given the importance and purposes of using stem cells in research and treatment, this technology cannot be easily ignored. This review provides an overview of the most recent articles in terms of knowing more about stem cell resources and their clinical applications.
Keywords: Stem Cell; Differentiation; Cell Type; Hematopoietic Stem Cells; Embryo Production
Introduction
What are stem cells?
Stem cells in multicellular organisms have the unique potential to divide and differentiate into a range of different cell types[1]. This unparalleled ability allows stem cells to play many roles, working together as an internal repair system, the ability to divide without restriction, allowing them to regenerate a variety of cells and tissues [2]. Since 1980, when stem cells were first isolated, scientists have been trying to understand their behavior and characteristics, hoping to change the treatment of people with hematological[3], oncological[4], dermatological[5-8], ophthalmological[9, 10], and orthopedic conditions[11]. Potential clinical applications have led to greater interest in the use of stem cells in many medical disciplines. The most important role that stem cells play in medicine is their therapeutic application because when cells are destroyed, damaged, or altered, they are replaced[12]. Advances in stem cell research have been made from time to time since their discovery, and the positive benefits have been enhanced by improving our understanding of their characteristics. There are serious ethical concerns about the use of stem cells, especially where embryos are destroyed to detect stem cell categories [13].
Embryonic and Adult stem cells
At the beginning of the 21st century, we have witnessed several experiments on the formation of mature stem cells and their various sources. In 2001, the first human embryo was cloned in the 4-6 cell stage to produce ESCs [14]. Because the production of these cells destroys blastocysts, ethical considerations have led research to other sources of stem cells[14, 15]. Various discoveries have been made since then, including spinal cord blood-derived embryonic stem cells (CBEs) that can differentiate into more cell types than mature stem cells and provide more important possibilities for cell-based therapies[16, 17]. In 2006, induced pluripotent stem cells (iPSCs) were introduced[18]. The ability to induce pluripotency has changed the field of stem cell research. First of all, it provides an alternative to ESCs, the main source of pluripotent cells. Second, it has been shown that the differentiating state of a cell can be manipulated, and third, a cell derived from an individual can be induced to become a type of cell capable of forming any other cell in that person’s body[19, 20]. Finally, iPSC from a specific person represents a highly personalized source of cells[21]. Another type of pluripotent stem cell was isolated from amniotic fluid in 2007, which is important because it can be used as a replacement for ESCs[22]. In 2010,the first ESC test was performed in the United States[23]. In 2002, the investment in stem cell research was boycotted because of moral problems with fetal death, but in 2009, it was allowed to continue[24]. Finally, a clinical trial was conducted by Grun Biotechnology Company. The company hoped to stimulate nerve growth in patients with spinal cord injury using GRNOPCI, a product derived from ESCs [25]. Although no functional results were reported from the trial, preliminary results were presented at the American Congress of Rehabilitation Medicine (ACRM) in October 2011. In the following years, various experiments and more clinical trials involving different types of stem cells were performed and continued. The genes commonly expressed in ESCs have been inserted into adult cells. A few mature cells become immature through this reprogramming and resemble embryonic stem cells with a condensation state[26].
Adult stem cells known as somatic stem cells are present in most but not all tissues and are often multipotent[27, 28]. There are some powerful adult stem cells that survive throughout life and, in response to damage, play a role in preserving and repairing the tissue seen there[29, 30]. They are found in many tissues, including the brain, bone marrow, blood vessels, heart, liver, and elsewhere, and are located in a specific area of each tissue called the stem cell niche [31-33].
Previously, iPSC cells were derived from urine[34], breast, and adipose tissue[35] by in vitro reprogramming of adult human cell resources in laboratories. The achievement of iPSCs has been possible by adding additional copies of the Oct4, Sox2, Klf4, and c-myc genes and following activation of the treated cells by feeding the mouse with the doxycycline antibiotic[36]. Gene expression profiling show iPSC cells have specific RNA patterns in part, in comparison to their counterpart ESCs but, the longer the iPSCs remain in the culture medium, the more similar they are to their ESC counterparts in terms of gene expression profile. These changes are not due to genomics changes, but in the reprogramming process, the expression activity of genes is affected by the culture environment and the epigenome [37]. Short-tail monkey angioblasts showed differentiation in 9 days cultures with different concentrations of BMP-4, FLT-3 ligand, stem cell factor, thrombopoietin, basal fibroblast growth factor. Real-time PCR results showed that ESC -derived angioblasts had downregulation in NANOG and OCT3/4 genes, but upregulation in T-brachyury and GATA2, and moderate expression of the CD34 gene. The CD144, TEK, or VWF genes do not express and differ in levels of CD13 expression [38, 39]. The recent investigators positively follow the ESC-derived angioplasty as promisingtherapeutic agents for repairing damaged arteries. In a different experience, a bioreactor was used to mimic damaged arteries from the isolated implanted cells from an animal and endothelial culture medium[40]. Researchers hope to regenerate specific cell types that could be a viable alternative to damaged organs.
Genetic alterations in stem cells to treat blood diseases such as beta-thalassemia
Sickle cell anemia and beta-thalassemia are the most common single-gene disorders worldwide, and about 317,000 newborns are affected each year [41]. Beta-thalassemia is caused by more than 200 different mutations in the β-globin gene that reduce or stop the production of β-globin chains[42]. For disease therapy, different trials using distinct vectors and genetic constructs under various regulations are performed. TNS9.3.55 lentivirus vector expressing the wild type β-globin transgenic gene was used to treat beta-thalassemia patients in the United States where, four treated patients did not show sufficient clinical benefits [43, 44]. Using the BB305 vector, scientists could increase injection efficiency after integrating the genome with HPV569 and the patients became independent of the injection more than 12 months after the gene therapy induction [45].
New methods of gene therapy for hematopoietic stem cells (HSC) emphasize the collection of cultured and expanded patient’s HSCs in an in vitro environment, modified using retroviral vector g or lentivirus, and re-injected into patients with myeloma with a set of disadvantages[46]. It seems that culturing HSCs in the presence of a mixture of cytokines hurts the long-term survival of the treated cells and their ability to establish and reprogramming. The manipulating HSCs in the ex vivo environment is challenging in terms of gene regulation[47]. Such gene therapy patterns become more expensive and severely restrict patient’s access to the treatments [48]. The ability of AAV vectors to effectively transform genomes into HSC nuclei has led to the development of gene targeting strategies[49]. Genetically modified ESCs using ZFN (Zinc finger nuclease) endonuclease and AAV6-derived vectors engineered in NSG mice showed long-term grafting and differentiation[50]. High tendency AAV6 to skeletal muscle immediately after intravascular injection, B cell and T cell responses against Ad/AAV capsid proteins, and the requirement to higher doses of gene therapy carriers compared to Ex vivo methods, are targeting barriers for gene therapy[51, 52]. The main factors converting fetal hemoglobin to adult hemoglobin are shown in Figure1[53]. Based on the reports, the connection areas of BCL11A in the β-globin gene may cause the HbF to shut down[53]. Recent treatment strategies are based on the use of 1) lentivirus vectors or 2) genome editing tools to reactivate endogenous HbF expression. The first strategy includes gene addition, reactivation of embryonic γ-globin gene expression by reducing BCL11A expression by shRNA[54], reactivation of embryonic γ-globin gene expression by manipulating the β-globin locus-related chromatin structure[55], regulation of decreased expression of α and βS-globin[56]. The second strategy includes gene modification, activation of fetal γ-globin gene expression by BCL11A expression reduction[57], reactivation of embryonic γ-globin gene expression by HPFH mutagenesis[58, 59], respectively.
Acquired aplastic anemia results from partial or complete destruction of the bone marrow. Cell therapy reports show that using HSCs has been effective in some patients with some degree of health improvement[60]. Therefore, many efforts have been made to change the conditions for the use of stem cells, especially mesenchymal stem cells to increase the effectiveness of treatment while experiencing less immune rejection. These cells are good candidates for anemia therapy due to the modulation of the immune system [61].
Stem cells and their genetic control in the treatment of infertility
World health organization (WHO) estimates 50–80 million infertile people worldwide[62]. The causes of male infertility remain unknown, but it is known that few genetic defects, such as some structural and numerical chromosomal abnormalities with deletions on the Y chromosome[63], some gonadotoxic drugs[64, 65], radiation[66, 67], chemotherapy [68] lead to male infertility[69].
The World Health Organization reports indicate that 37% of couples’ infertility is due to female factors[70], most of which are related to ovarian disease (26%)[71] and endometriosis (10-15%)[72]. Stem cell therapy has been widely suggested for the treatment of infertility in women for ovarian regeneration and egg production [73]. Some sources of active mitotic germ cells that can be purified and cultured in vitro to spontaneously form oocytes or eggs have been reported in human ovaries [74, 75]. This suggests that women of reproductive age may have follicular reserves without genetic defects. Therefore, it seems that the reproductive capacity could be maintained by stem cell therapy of azoospermic men [76] and improve fertility in women[77]. Moreover, stem cell transplantation could repair the loss of fertility in women due to radiotherapy or chemotherapy and retain women’s ovarian ability[78]. Table 1 shows the characteristics of most of the stem cells used to treat infertility.
Today, different stem cells which vary in strength and proliferation including ESCs[79], MSCs[80], extra- ESCs [81], iPSCs[82], SSC [83, 84], and PGCs are used to treat infertility[81, 85]. Characteristics of stem cells and recent advancements in the treatment of infertility are shown in Table1.
Genetic expression profile of cancer stem cells for predicting treatment
Cancer is a heterogeneous population of different cells with different phenotypic and functional properties that lead to therapeutic outcome limitations. Cancer stem cells (CSCs) are found in tumor tissues just as natural stem cells are found in normal tissues. There is plentiful evidence that CSCs are caused by any mutation in natural progenitors of stem cells, or by an abnormality in the genetic routes that regulate these cells.
CSCs are protected by multiple stable mechanisms that lead to tumor metastasis, therapeutic resistance, and recurrence[86]. Thus, targeted CSC therapies represent a promising strategy for the long-term treatment of the disease[87]. There are common signaling pathways between stem cells (SCs) and CSCs, including 1) JAK / STAT, 2) Hedgehog, 3) Wnt, 4) Notch, 5) PTEN / AKT / P13K, 6) NF-κB, MAPK / ERK, and SMAD pathways[88].
Tumor microenvironments play a key role in regulating the CSC phenotype [89]. Hence, cancer-related fibroblasts increase tumor growth and retain the basic properties of CSCs in the form of paracrine in different types of cancer[90]. Also, adipocytes, which increase the absorption of inflammatory cells, especially macrophages, by secreting various adipokines and cytokines such as leptin, adiponectin, IL-6, MCP-1, and TNF-a, and cause chronic inflammation for cancer growth and metastasis [91, 92]. In addition, perivascular cells in angiogenesis[93], tumor-associated macrophages[94], myeloid suppressor cells (MDSCs)[95], regulatory T cells[96], natural killer cells[97], mast cells (MCs)[98], all play an important role in regulating the CSC population.
The hypoxia as a microenvironment factor that is affected by the expression of the hypoxia gene under the hypoxic induction factors including HIF-1α[99, 100], HIF-2α transcription[101], and the other gene inducers that bind to gene promoters’ HRE (hormone response element) exacerbate CSC invasion (Figure 1). In addition to invasion, hypoxia contributes to drug resistance by keeping CSCs silent and helps in chemotherapy resistance, which usually targets the active division of cancer cells[102]. Moreover, extracellular matrix (ECM) by altering their dynamics and binding to CSCs by their receptors, keep them in a proliferative state[103].
Various experiments have been examined the expression of CSCs related genes to introduce a candidate gene as a predictive biomarker[104]. Interestingly, baseline expression of CSC-related genes, including Wnt basal signaling, predicted high efficacy ONC201 anti-cancer in more than 1,000 cancer cell lines [105, 106]. Still, various interdependent studies are testing CSCs’ RNA and protein expression, using circulating tumor cells and biopsies obtained from ONC201 clinical trials.
Application of mesenchymal stem cells in the treatment of brain tumors and effective genes profile in their differentiation.
Gliomas are the most common tumors of the central nervous system that are highly invasive and impose a heavy economic burden worldwide[107]. Malignant Gliomas include anaplastic astrocytoma and glioblastoma. According to the WHO classification system grade, IV astrocytoma is the most common and deadly primary brain tumor in adults[108].
Investigations show that effective targeted therapies in cancers are less responsive to brain tumors[109]. Glioblastoma contains glioma-like stem cells that make them resistant to most treatments[110]. Based on a recent theory, the disease recurrence is caused by glioma stem cells that appear to make the tumor resistant to radiation and chemotherapy [111-113].
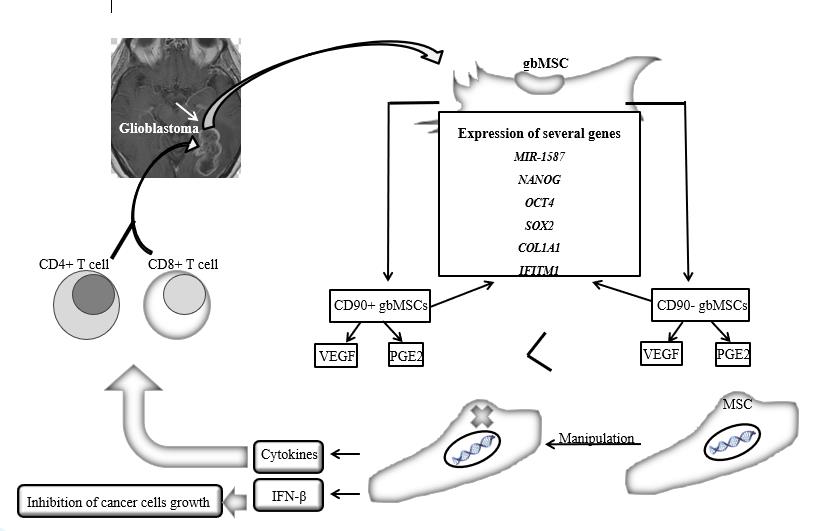
Glioma-associated mesenchymal stem cells (gbMSCs) were first isolated from fresh glioma tissue in 2014 by Zhang et al. [114]. They express different factors depending on the intracellular condition or the hypoxia condition[115]. The percentage of gbMSCs is higher in therapy-resistant tumor specimens, therefore, the patients are less likely to survive[116]. CD90− gbMSCs produce more VEGF and prostaglandin E2 (PGE2) than CD90+ cells[117]. The expression of several genes such as miR-1587, Nanog[118] and OCT4, SOX2 [119, 120], COL1A1[121], and IFITM1[122], have been measured in these two subsets of gbMSCs, indicating the involvement of these cells in tumorigenesis and invasion.
The propensity of MSCs for tumors and their ability to cross the blood-brain barrier have identified MSC cells as transporters for the treatment of glioma [123]. The genetically modified MSCs could secret soluble protein IFN-β, which dramatical ly increases the survival of animals with intracranial gliomas and inhibits the growth of tumor cells in a dose-related manner [124, 125]. The results show that the genetically engineered MSCs can express cytokines and boost the immune system by improving the penetration of CD4 + and CD8 + T-cells and stimulating the cascading immune network[126, 127]. Glioblastoma treatment with cellular suicide proteins such as thymidine kinase[128], herpes simplex virus (HSV-TK)[129], cytosine deaminase/5-fluorocytosine (CD/5FC), rabbit carboxyl ester (rCE)/CPT-11, HSV is experienced from which system TK/GCV is the most successful in treating glioma[130], (Figure 2).
Oncolytic virus therapy is a new approach in which viruses are genetically engineered to selectively replicate in tumor cells[131]. Treatment based on cytotoxic factors, anti-angiogenic therapies[132], and microRNA transfection by MSC [133] are other therapy methods to treat the disease. Important challenges in the treatment of glioblastoma include malignant MSCs and immunosuppression by MSCs.
Strategies for differentiating tooth pulp stem cells into neurons and genes influencing this pathway.
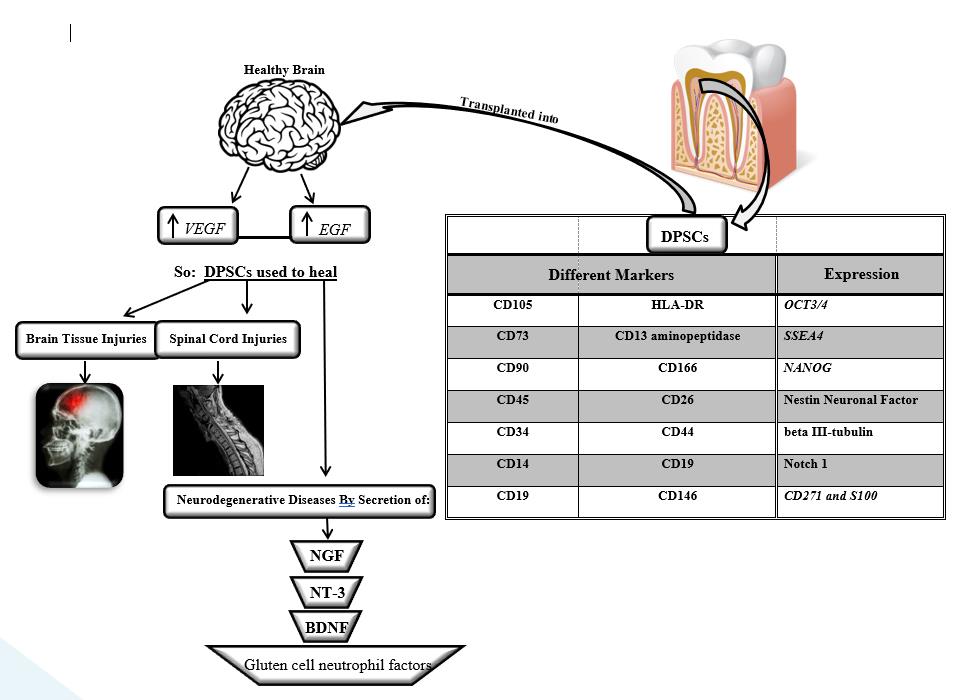
Dental pulp and tooth-supporting tissue originate from the cranial neural crest. Several studies have reported the separation of the cell population from after birth dental pulp tissue, which has clonogenic, plasticity (flexibility), and multivariate capabilities, and is therefore called dental pulp stem cells (DPSCs)[134]. These cells are identified by different markers such as CD105 (endoglin), CD73 (5-ectonucleotidase), and CD90 (Thy-1) CD45, CD34, CD14 or CD11-b, CD79a or CD19 and HLA-DR, CD13 aminopeptidase, CD26, CD44, CD44,CD166, and CD146[135]. The expression of OCT3/4, SSEA4 stem cell factors and NANOG, expression of nestin neuronal factor, beta III-tubulin, S100, Notch 1, CD271, and some other important factors are also seen in these cells[136].
Transplanted DPSCs into a healthy undamaged brain, stimulated the proliferation and migration of endogenous neurons, and enhanced the expression of neurodegenerative factors such as VEGF and EGF at the site of transplantation [137, 138]. Although the transplant itself is short-lived, these results indirectly highlight the ability of DPSCs to regulate brain tissue as a good choice for brain injuries such as brain trauma and stroke, treatment of spinal cord injuries, and retinal repair and treatment of eye injuries [139, 140]. Moreover, DPSCs exhibit neuroprotective and regenerative properties through the paracrine mechanism due to the secretion of neurotrophic factors such as NGF, NT-3, BDNF, and gluten cell neutrophil factors [141, 142], (Figure 3).
Stem cells and organ regeneration, requirements, systems, methods, successes, and problems
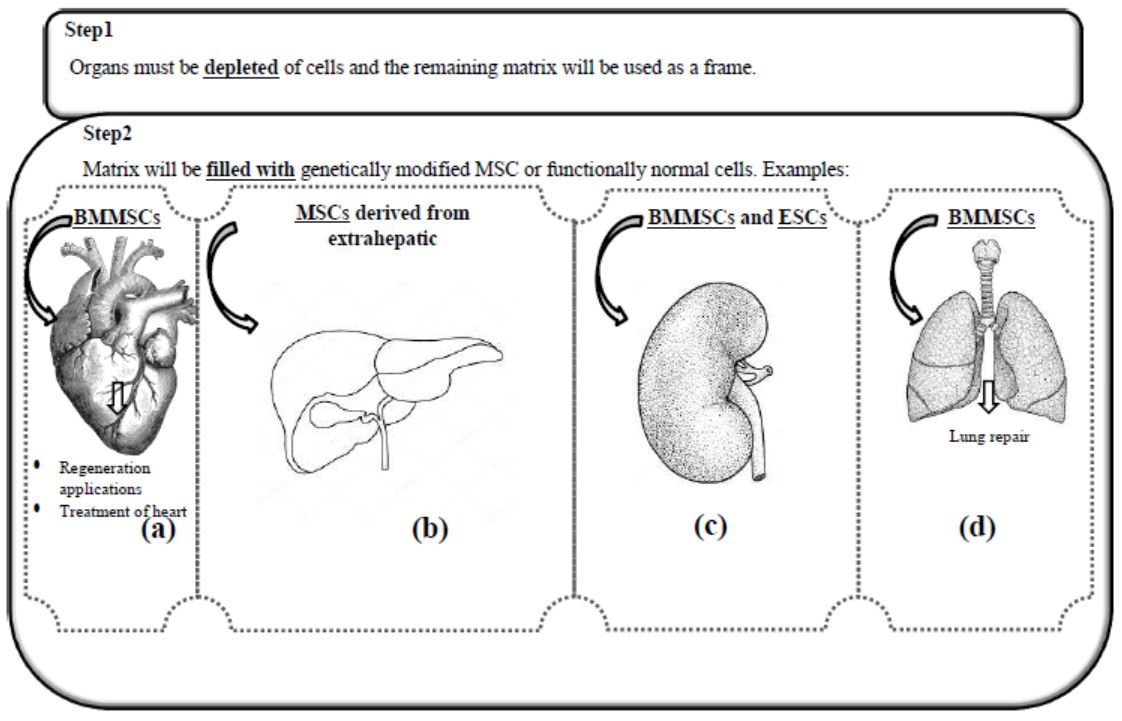
translationOrgan reconstruction is of great vital importance for the elderly, the injured, and also for the congenitally disabled people. Technically, the required organ, such as the heart or kidney, must be emptied of cells, the remaining cellular network or matrix to be used as a frame, and genetically modified stem cells or cells with normal functions will fill the matrix instead[143]. Of course, it is unlikely to reconstruct the complex structures of an organ made up of different cell types, parts, vessels, and nerves[144]. In this regard, the presence of a bioreactor for continuous oxygenation, a specific culture medium containing growth factors, and other adhesion and/or cell growth factors is essential[145].
Construction of the heart as an alternative to the treatment of heart failure using bone marrow mesenchymal stem cells (BMMSC) and biological materials for regeneration[146], liver construction using MSCs derived from extrahepatic and bio-supporting materials[147, 148], Lung repair using multipotential stem cells such as BMMSC[149], and the construction of the kidney with that complex tissue structure and the heterogeneous nature of cells using ESCs and BMMSC, has been well performed in numerous in-vitro and ex-vivo experiments and is being optimized to complete success[150], (Figure 4).
Discussion
The development of stem cell-based therapies and their applications in regenerative medicine has become increasingly dependent on animal models[151]. The results of these models helped to better understand the mechanisms of cell therapy [2]. However, large animal models have shown the better translationOrganal ability for benefit of humans, as much evidence has shown a clear difference between mouse and human ESCs [152, 153]. It is not yet clear what percentages of iPSCs and ESCs’ gene expression profiles are similar, and whether different iPSCs from different cell lines differ in this respect and whether this difference has a significant effect on cell biology and function[154]. Answering these questions increases the risk of success in using ESCs or IPSCs for tissue or organ engineering.
Conclusion
Short-tailed monkeys (Baboons) have an advantage over other models due to their high level of resemblance in physiological traits to humans, which contribute to the development of clinical applications of stem cell therapies[155]. The Baboon-to-human similarity in the kinetics and effect of CD34 + / CD31 + cells, in the expression pattern of kinase insertion domain receptor (KDR)[156], in differentiation into several races such as vascular cell lines, and compensatory function in repairing damaged vascular cells in monkeys and a similar function in human stem cells are key to identifying the molecular and cellular events that regulate and determine the fate of the fetal ECs in human stem cells[157, 158].
Also, the complete replacement of human organs with another organ from a matrix made of animal origin- bearing cells capable of producing an organ with efficient mechanical activity is a distant prospect that is likely to occur in the next two decades.
Declaration
This study was approved by Shahid Beheshti University of Medical Sciences ethics committee.
- Biehl JK, B Russell (2009) Introduction to stem cell therapy. The Journal of cardiovascular nursing 24: 98-105.
- Zakrzewski W (2019) Stem cells: past, present, and future. Stem cell research & therapy 10: 68.
- Wattanapanitch M (2019) Recent Updates on Induced Pluripotent Stem Cells in Hematological Disorders. Stem Cells International 2019: 5171032.
- Hawsawi YM (2018) Stem Cell Applications for Treatment of Cancer and Autoimmune Diseases: Its Promises, Obstacles, and Future Perspectives. Technology in cancer research & treatment 17: 1533033818806910.
- Ogliari KS (2014) Stem cells in dermatology. Anais brasileiros de dermatologia 89: 286-91.
- Shpichka A (2019) Skin tissue regeneration for burn injury. Stem Cell Research & Therapy 10: 94.
- Maguire G (2019) The safe and efficacious use of secretome from fibroblasts and adipose-derived (but not bone marrow-derived) mesenchymal stem cells for skin therapeutics. J clin aesthetic dermatol 12: E57.
- Tan ST R Dosan (2019) Lessons From Epithelialization: The Reason Behind Moist Wound Environment. The Open Dermatology J 13: 34-40.
- O’Callaghan AR, JT Daniels (2011) Concise review: limbal epithelial stem cell therapy: controversies and challenges. Stem cells 29: 1923.
- Aharony I, S Michowiz, N Goldenberg-Cohen (2017) The promise of stem cell-based therapeutics in ophthalmology. Neural Regeneration Res 12: 173.
- Maniar H (2015) The current role of stem cells in orthopaedic surgery. Malaysian orthopaedic J 9: 1.
- Mahla RS (2016) Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int J cell biol 2016: 6940283.
- Volarevic V (2018) Ethical and Safety Issues of Stem Cell-Based Therapy. Int J med sci 15: 36-45.
- Cibelli JB (2002) The first human cloned embryo. Scientific Ame 286: 44-51.
- Hug K (2011) Embryonic stem cell research: an ethical dilemma. EuroStemCell.
- Nandoe Tewarie RS (2009) Stem cell-based therapies for spinal cord injury. The journal of spinal cord med 32: 105-14.
- Salewski RP (2015) Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem cells and development 24: 36-50.
- Sirinathinghji E (2011) The promise of induced pluripotent stem cells. Sci Soc 51: 42-3.
- Medvedev SP, AI Shevchenko, SM Zakian (2010) Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta naturae 2: 18-28.
- Omole AE, AOJ Fakoya (2018) Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 6: e4370-e4370.
- Ha, H.-Y, SH Jang, JW Jung (2011) The use of pluripotent stem cell for personalized cell therapies against neurological disorders. J biomed biotech 2011: 520816.
- Baghaban Eslaminejad M, S Jahangir (2012) Amniotic fluid stem cells and their application in cell-based tissue regeneration. International journal of fertility & sterility 6: 147-56.
- Eguizabal C (2019) Two decades of embryonic stem cells: a historical overview. Human reproduction open 2019: hoy024.
- Wolinsky H (2009) The pendulum swung. President Barack Obama removes restrictions on stem-cell research, but are expectations now too high? EMBO reports 10: 436-9.
- Stein GS (2011) Human stem cell technology and biology: A research guide and laboratory manual. 2011: John Wiley & Sons.
- Orqueda AJ, CA Giménez, F Pereyra-Bonnet (2016) iPSCs: A Minireview from Bench to Bed, including Organoids and the CRISPR System. Stem Cells Int 2016: 5934782.
- Magli MC, E Levantini, A Giorgetti (2004) Developmental potential of somatic stem cells in mammalian adults. J Hematotherapy & Stem Cell Res 9: 6.
- Blau HM, T Brazelton, J Weimann (2001) The evolving concept of a stem cell: entity or function? Cell 105: 829-41.
- Baraniak PR, TC McDevitt (2010) Stem cell paracrine actions and tissue regeneration. Regenerative med 5: 121-43.
- Lane SW, DA Williams, FM Watt (2014) Modulating the stem cell niche for tissue regeneration. Nature biotechnol 32: 795-803.
- De Cuevas M, EL Matunis (2011) The stem cell niche: lessons from the Drosophila testis. Development 138: 2861-9.
- Ferraro F, CL Celso, D Scadden (2010) Adult stem cels and their niches. Advances in experimental medicine and biol 695: 155-68.
- Pennings S, KJ Liu, H Qian (2018) The Stem Cell Niche: Interactions between Stem Cells and Their Environment. Stem Cells Int 2018: 4879379.
- Manaph NPA (2018) Urine-derived cells for human cell therapy. Stem cell research & therapy 9: 189.
- Wu L (2019) The Therapeutic Potential of Adipose Tissue-Derived Mesenchymal Stem Cells to Enhance Radiotherapy Effects on Hepatocellular Carcinoma. Frontiers in Cell and Developmental Biol 2019: 7.
- Choi HW (2015) In vivo reprogrammed pluripotent stem cells from teratomas share analogous properties with their in vitro counterparts. Scientific rep 5: 13559.
- Chin MH (2009) Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell stem cell 5: 111-23.
- Shi Q (2013) Ex vivo reconstitution of arterial endothelium by embryonic stem cell-derived endothelial progenitor cells in baboons. Stem Cells Dev 22: 631-42.
- Richter M (2017) In Vivo Hematopoietic Stem Cell Transduction. Hematol Oncol Clin North Am, 2017. 31(5): p. 771-85.
- Shi Q (2013) Ex vivo reconstitution of arterial endothelium by embryonic stem cell-derived endothelial progenitor cells in baboons. Stem cells and development 22: 631-42.
- De Sanctis V (2017) β-Thalassemia Distribution in the Old World: an Ancient Disease Seen from a Historical Standpoint. Mediterranean J hematology and infectious diseases 9: e2017018.
- Hassan T (2016) β-Thalassemia: Genotypes and Phenotypes. Epidemiology of Communicable and Non-Communicable Diseases-Attributes of Lifestyle and Nature on Humankind, 2016: 113-26.
- Karponi G, N Zogas (2019) Gene Therapy For Beta-Thalassemia: Updated Perspectives. The application of clinical genetics 12: 167-80.
- Mansilla-Soto J (2016) Cell and Gene Therapy for the Beta-Thalassemias: Advances and Prospects. Human gene therapy 27: 295-304.
- Cavazzana M, F Mavilio (2018) Gene Therapy for Hemoglobinopathies. Human gene therapy 29: 1106-13.
- Morgan RA (2017) Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell stem cell 21: 574-90.
- Tajer P (2019) Ex Vivo Expansion of Hematopoietic Stem Cells for Therapeutic Purposes: Lessons from Development and the Niche. Cells 8: 169.
- Salzman R (2018) Addressing the Value of Gene Therapy and Enhancing Patient Access to Transformative Treatments. Molecular therapy : the journal of the American Society of Gene Therapy 26: 2717-26.
- Alexander IE, DW Russell (2015) The Potential of AAV-Mediated Gene Targeting for Gene and Cell Therapy Applications. Current Stem Cell Rep 1: 16-22.
- Lee J (2020) Recent advances in genome editing of stem cells for drug discovery and therapeutic application. Pharmacology & Therapeutics 209: 1.
- Mingozzi F, KA (2013) High, Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122: 23-36.
- Naso MF (2017) Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy 31: 317-34.
- Cavazzana M, C Antoniani, A Miccio (2017) Gene therapy for β-hemoglobinopathies. Molecular Therapy, 2017. 25: 1142-54.
- Cavazzana M, C Antoniani, A Miccio (2017) Gene Therapy for β-Hemoglobinopathies. Molecular therapy : the journal of the American Society of Gene Therapy 25: 1142-54.
- Deng W (2014) Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell 158: 849-60.
- Mettananda S, RJ Gibbons, DR Higgs (2016) Understanding α-globin gene regulation and implications for the treatment of β-thalassemia. Annals of the New York Academy of Sci 1368:16-24.
- Suzuki M, M Yamamoto, JD Engel (2014) Fetal globin gene repressors as drug targets for molecular therapies to treat the β-globinopathies. Molecular and cellular biol 34: 3560-9.
- Bauer DE, SH Orkin (2011) Update on fetal hemoglobin gene regulation in hemoglobinopathies. Current opinion in pediatrics 23: 1-8.
- Zhan J (2020) High level of fetal-globin reactivation by designed transcriptional activator-like effector. Blood advances 4: 687-95.
- Medinger M (2018) Pathogenesis of Acquired Aplastic Anemia and the Role of the Bone Marrow Microenvironment. Front Oncol 8: 587.
- Han Y (2019) Mesenchymal Stem Cells for Regenerative Medicine. Cells 8: 1.
- Babakhanzadeh E (2020) Some of the Factors Involved in Male Infertility: A Prospective Review. International J general med 13: 29-41.
- Arafa MM (2018) Chromosomal abnormalities in infertile men with azoospermia and severe oligozoospermia in Qatar and their association with sperm retrieval intracytoplasmic sperm injection outcomes. Arab J urology 16: 132-9.
- Samplaski MK, AK Nangia (2015) Adverse effects of common medications on male fertility. Nature Reviews Urology 12: 401-3.
- Semet M (2017) The impact of drugs on male fertility: a review. Andrology 5: 640-63.
- Kesari KK, A Agarwal, R Henkel (2018) Radiations and male fertility. Reproductive biology and endocrinology 16: 118.
- De Felice F (2019) Radiation effects on male fertility. Andrology 7: 2-7.
- Okada K, M Fujisawa (2019) Recovery of Spermatogenesis Following Cancer Treatment with Cytotoxic Chemotherapy and Radiotherapy. The world J men’s health 37: 166-74.
- Hamada A, SC Esteves, A Agarwal (2011) Unexplained male infertility: potential causes and management. Human Andrology 1: 2-16.
- WHO (1992) Recent advances in medically assisted conception. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser 820: 1-111.
- Goodman NF (2015) American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Exess and PCOS Society Dosease State Clinical Review: Guide to the best practices in the evaluation and treatment of Polycistic Ovary Syndrome--part1. Endocr Pract 21: 1291-300.
- Parasar P, P Ozcan, KL (2017) Terry, Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr Obstet Gynecol Rep 6: 34-41.
- Wang J (2019) Stem Cells as a Resource for Treatment of Infertility-related Diseases. Curr Mol Med 19: 539-46.
- Hanna CB, JD Hennebold (2014) Ovarian germline stem cells: an unlimited source of oocytes? Fertil Steril 101: 20-30.
- Telfer E, R Anderson (2019) The existence and potential of germline stem cells in the adult mammalian ovary. Climacteric 22: 22-6.
- Ibtisham F, A Honaramooz (2020) Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility. Cells 9: 745.
- Vermeulen M (2019) Role of stem cells in fertility preservation: current insights. Stem cells and cloning : advances and applications 12: 27-48.
- Akahori T, DC Woods, JL Tilly (2019) Female fertility preservation through stem cell-based ovarian tissue reconstitution in vitro and ovarian regeneration in vivo. Clinical Medicine Insights: Reproductive Health 13: 1179558119848007.
- Lee Y, E Kang (2019) Stem cells and reproduction. BMB Rep 52: 482-9.
- Zhao YX (2019) Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem Cells International.
- Wang J (2019) Stem Cells as a Resource for Treatment of Infertility-related Diseases. Current molecular medicine 19: 539-46.
- Fang F (2018) Human induced pluripotent stem cells and male infertility: an overview of current progress and perspectives. Human reproduction (Oxford, England) 33: 188-95.
- Izadyar F (2003) Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction 126: 765-74.
- Ibtisham F, AH Awang-Junaidi, A Honaramooz (2020) The study and manipulation of spermatogonial stem cells using animal models. Cell and Tissue Res 380: 393-414.
- Dakhore S, B Nayer, K Hasegawa (2018) Human pluripotent stem cell culture: current status, challenges, and advancement. Stem cells int 2018: 17
- De Angelis ML, F Francescangeli, A Zeuner (2019) Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 11: 1569.
- Yang T, K Rycaj (2015) Targeted therapy against cancer stem cells. Oncology letters 10: 27-33.
- Aponte PM, A Caicedo (2017) Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment. Stem Cells Int 2017: 5619472.
- Ayob AZ, TS Ramasamy (2018) Cancer stem cells as key drivers of tumour progression. J biomed sci 25: 20.
- Fiori ME (2019) Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Molecular cancer 18: 70.
- Divella R (2016) Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. Journal of Cancer 7: 2346-59.
- Yazdanpanah B (2018) Adipokine Role in Normal and Neoplastic Bone Marrow Niche. Clinical Cancer Investigation J 7: 37.
- Lau EYT, NPY Ho, TKW Lee (2017) Cancer Stem Cells and Their Microenvironment: Biology and Therapeutic Implications. Stem Cells Int 2017: 3714190.
- Ravindran S, S Rasool, C Maccalli (2019) The Cross Talk between Cancer Stem Cells/Cancer Initiating Cells and Tumor Microenvironment: The Missing Piece of the Puzzle for the Efficient Targeting of these Cells with Immunotherapy. Cancer Microenvironment 12: 133-48.
- Ai L (2019) Myeloid-derived suppressor cells endow stem-like qualities to multiple myeloma cells by inducing piRNA-823 expression and DNMT3B activation. Molecular Cancer 18: 88.
- Yang L (2020) Targeting cancer stem cell pathways for cancer therapy. Signal Transduction and Targeted Therapy 5: 8.
- Luna JI (2017) Targeting cancer stem cells with natural killer cell immunotherapy. Expert opinion on biological therapy 17: 313-24.
- Krystel-Whittemore M, KN Dileepan, JG Wood (2016) Mast Cell: A Multi-Functional Master Cell. Frontiers in Immunology 6: 1.
- Semenza GL (2010) Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29: 625-34.
- Lee SY (2018) Regulation of Tumor Progression by Programmed Necrosis. Oxidative Medicine and Cellular Longevity 2018: 3537471.
- Gilkes DM (2016) Implications of Hypoxia in Breast Cancer Metastasis to Bone. Int J molecular sci 17: 1669.
- Phi LTH (2018) Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem cells int 2018: 5416923.
- Nallanthighal S, JP Heiserman, DJ Cheon (2019) The Role of the Extracellular Matrix in Cancer Stemness. Frontiers in cell and developmental biol 7: 86.
- Jiang P (2018) Genome-scale signatures of gene interaction from compound screens predict clinical efficacy of targeted cancer therapies. Cell systems 6: 343-54
- Prabhu VV (2017) Cancer stem cell-related gene expression as a potential biomarker of response for first-in-class imipridone ONC201 in solid tumors. PloS one 12: e0180541.
- Yang Y (2020) Emerging agents that target signaling pathways in cancer stem cells. Journal of Hematology & Oncology 13: 60.
- Araghi M (2020) Incidence of Malignant Brain and Central Nervous System Tumors in Golestan, Iran, 2004–2013. Archives of Iranian med 23: 1-6.
- Hanif F (2017) Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pacific journal of cancer prevention: APJCP 18: 3-9.
- Jain KK (2018) A Critical Overview of Targeted Therapies for Glioblastoma. Frontiers in oncology, 2018. 8: 419.
- Garnier D (2019) Glioblastoma Stem-Like Cells, Metabolic Strategy to Kill a Challenging Target. Frontiers in Oncology 9: 1-18.
- Liu Y (2020) Radiotherapy targeting cancer stem cells “awakens” them to induce tumour relapse and metastasis in oral cancer. Int J Oral Sci 12: 1-19.
- Sachdeva R (2019) BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma. Scientific Rep 9: 14569.
- Kostopoulou ON (2018) Glucocorticoids promote a glioma stem cell‐like phenotype and resistance to chemotherapy in human glioblastoma primary cells: Biological and prognostic significance. International Journal of Cancer 142: 1266-76.
- Zhang Q (2018) CD90 determined two subpopulations of glioma-associated mesenchymal stem cells with different roles in tumour progression. Cell Death & Dis 9: 1101.
- Zhang Q (2018) Current status and potential challenges of mesenchymal stem cell-based therapy for malignant gliomas. Stem Cell Research & Therapy 9: 228.
- Shahar T (2017) Percentage of mesenchymal stem cells in high-grade glioma tumor samples correlates with patient survival. Neuro-oncology 19: 660-8.
- Svensson A (2017) Identification of two distinct mesenchymal stromal cell populations in human malignant glioma. Journal of neuro-oncology 131: 245-54.
- Patra SK (2018) Molecular characterization and expression patterns of Nanog gene validating its involvement in the embryonic development and maintenance of spermatogonial stem cells of farmed carp, Labeo rohita. J Animal Science and Biotechnol 9: 45.
- Ruiz G (2019) Genes Involved in the Transcriptional Regulation of Pluripotency Are Expressed in Malignant Tumors of the Uterine Cervix and Can Induce Tumorigenic Capacity in a Nontumorigenic Cell Line. Stem Cells Int 2019: 7683817.
- Lopez-Bertoni H (2016) Epigenetic modulation of a miR-296-5p:HMGA1 axis regulates Sox2 expression and glioblastoma stem cells. Oncogene 35: 4903-13.
- Sun S (2018) Identification of COL1A1 as an invasion‑related gene in malignant astrocytoma. Int J oncology 53: 2542-54.
- Ogony J (2016) Interferon-induced transmembrane protein 1 (IFITM1) overexpression enhances the aggressive phenotype of SUM149 inflammatory breast cancer cells in a signal transducer and activator of transcription 2 (STAT2)-dependent manner. Breast cancer research: BCR 18: 25.
- Kwon S (2019) Mesenchymal stem cell therapy assisted by nanotechnology: a possible combinational treatment for brain tumor and central nerve regeneration. Int J nanomed 14: 5925-42.
- Sage EK, RM Thakrar, SM Janes (2016) Genetically modified mesenchymal stromal cells in cancer therapy. Cytotherapy 18: 1435-45.
- Chulpanova DS (2018) Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Frontiers in Pharmacol 9: 1-10.
- Rawat S, S Gupta, S Mohanty (2019) Mesenchymal stem cells modulate the immune system in developing therapeutic interventions, in Immune Response Activation and Immunomodulation. IntechOpen 2019: 1-24.
- Hadryś A (2020) Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. European J Pharmacol 874: 172991.
- Chao CN (2018) Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Scientific Rep 8: 2213.
- Okura H, CA Smith, JT Rutka (2014) Gene therapy for malignant glioma. Molecular and Cellular Therapies 2: 21.
- Tamura R (2019) Recent progress in the research of suicide gene therapy for malignant glioma. Neurosurgical Review 2049: 29-49.
- Shi T (2020) Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment. Frontiers in Immunol 11: 1-13.
- Javan MR, A Khosrojerdi, SM Moazzeni (2019) New Insights Into Implementation of Mesenchymal Stem Cells in Cancer Therapy: Prospects for Anti-angiogenesis Treatment. Frontiers in oncol 9: 840.
- Lang, FM (2018) Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-oncology 20: 380-90.
- Orimoto A (2020) Efficient immortalization of human dental pulp stem cells with expression of cell cycle regulators with the intact chromosomal condition. Plos one 15: e0229996.
- Ledesma-Martínez E, VM Mendoza-Núñez, E Santiago-Osorio (2016) Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem cells int 4709572.
- Atari M (2011) Isolation of pluripotent stem cells from human third molar dental pulp. Histol Histopathol 26: 1057-70.
- Zong X (2017) Transplantation of VEGF-mediated bone marrow mesenchymal stem cells promotes functional improvement in a rat acute cerebral infarction model. Brain Res 1676: 9-18.
- Gancheva MR (2019) Using Dental Pulp Stem Cells for Stroke Therapy. Frontiers in Neurology 10: 1-17.
- Wu S, KT FitzGerald, J Giordano (2018) On the Viability and Potential Value of Stem Cells for Repair and Treatment of Central Neurotrauma: Overview and Speculations. Frontiers in neurology 9: 602.
- Kimbrel EA, R Lanza (2020) Next-generation stem cells—ushering in a new era of cell-based therapies. Nature Reviews Drug Discovery 2020: 1-17.
- Pagella P (2020) Human dental pulp stem cells exhibit enhanced properties in comparison to human bone marrow stem cells on neurites outgrowth. The FASEB J 34: 5499-511.
- Ueda T (2020) Characteristics and Therapeutic Potential of Dental Pulp Stem Cells on Neurodegenerative Diseases. Frontiers in Neuroscience 14: 1.
- Mandrycky C, K Phong, Y Zheng (2017) Tissue engineering toward organ-specific regeneration and disease modeling. MRS communications 7: 332-47.
- Zhang B (2019) 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering 5: 777-94.
- Petcavich RJ (2017) Method of manufacturing or differentiating mammalian pluripotent stem cellsor progenitor cells using a hollow fiber bioreactor. Google Patents.
- Taylor DA (2019) The Future of Tissue Engineering in Heart Transplantation. Texas Heart Institute J 46: 73-4.
- Skeldon G, B Lucendo-Villarin, W Shu (2018) Three-dimensional bioprinting of stem-cell derived tissues for human regenerative medicine. Philosophical transactions of the Royal Society of London. Series B, Biological sci 373: 20170224.
- Rossi EA (2019) Advances in Hepatic Tissue Bioengineering with Decellularized Liver Bioscaffold. Stem Cells Int 2019: 2693189.
- Fang S (2019) The role of pulmonary mesenchymal cells in airway epithelium regeneration during injury repair. Stem Cell Research & Therapy 10: 366.
- Rak-Raszewska A, PV Hauser, S Vainio (2015) Organ In Vitro Culture: What Have We Learned about Early Kidney Development? Stem Cells Int 2015: 959807. 151. Nawab K (2019) Stem Cell Therapies: A Way to Promising Cures. Cureus 11: e5712.
- Nawab K (2019) Stem Cell Therapies: A Way to Promising Cures. Cureus 11: e5712.
- Kim J, BK Koo, JA Knoblich (2020) Human organoids: model systems for human biology and medicine. Nature Reviews Molecular Cell Biol 21: 571-84.
- Harding J, RM Roberts, O Mirochnitchenko (2013) Large animal models for stem cell therapy. Stem cell research & therapy 4: 23.
- Hoffmann A, M Ziller, D Spengler (2020) Focus on Causality in ESC/iPSC-Based Modeling of Psychiatric Disorders. Cells 9: 366.
- Grow DA, JR McCarrey, CS Navara (2016) Advantages of nonhuman primates as preclinical models for evaluating stem cell-based therapies for Parkinson’s disease. Stem cell res 17: 352-66.
- Cox LA (2013) Baboons as a Model to Study Genetics and Epigenetics of Human Disease. ILAR J 54: 106-21.
- Olivier EN (2019) Differentiation of Baboon (Papio anubis) Induced-Pluripotent Stem Cells into Enucleated Red Blood Cells. Cells 8: 1282.
- Wu FL (2019) A comparison of humans and baboons suggests germline mutation rates do not track cell divisions. bioRxiv 2019: 844910.

Tables at a glance
Figures at a glance