Insights into the Role of Biodegradable Polymers and Stem Cells in Cardiac Tissue Regeneration
Received Date: February 23, 2023 Accepted Date: March 23, 2023 Published Date: March 27, 2023
doi: 10.17303/jscr.2023.5.101
Citation: Mai Abdelgawad(2023) Insights into the Role of Biodegradable Polymers and Stem Cells in Cardiac Tissue Regeneration.J Stem Cell Rep 5: 1-23
Abstract
Tissue engineering introduced a new landscape for regenerative medicine. It displays a distinctive feature for tissue regeneration in organs that lack the capabilities to regenerate themselves, particularly the heart. Adult mammals cannot compensate for the injured and dead cardiomyocytes. Instead, scar tissue is formed leading to a loss in cardiac contractile activity. Therefore,scaffold fabrication plays a substantial role to enhance cell regeneration and differentiation via utilizing biomaterials to mimic the natural tissue niche. One of the most significant biomaterials is biodegradable polymers which serve as a temporary scaffold to allow cells to regenerate and proliferate. Moreover, they provide the scaffold with mechanical strength. They also allow long-term biocompatibility with avoidance of surgery performance in order to remove the cardiac patch or scaffold.In this review, we provide an overview of biodegradable polymers used in cardiac tissue regeneration clarifying the most significant biodegradable polymers used in cardiac patches and scaffolds. We will discuss how these polymers facilitate stem cell delivery, engraftment, differentiation, and proliferation. In addition to summarizing the classification, properties, pros, and cons of biodegradable polymers.
Keywords: Biodegradable Polymers; Scaffolds; Stem Cells; Cardiac Regeneration; Tissue Engineering; Cardiomyocytes; Myocardial Infarction
AbbreviationsAniline tetramer-AT, Blood outgrowth endothelial cells –BOECs, Carbon nanotubes-CNTs, Cholesterol-modified PURPUR-Chol, Decellularized extracellular matrix-dECM, 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide-EDC, Embry onic stem cells-ESCs, Endothelial progenitor cells-EPCs, Engelbreth-Holm-Swarm-EHS, Extracellular matrix-ECM, Hematopoietic stem cells-HSCs, Hydroxysuccinimide-NHS, induced pluripotent stem cells-iPSCs, Laminin-1-LN1, Matrix metalloproteinase 9-MMP9, Mesenchymal stromal cells-MSCs, Placental growth factor-PlGF, Platelet-derived growth factor-BB-DGF-BB, Pluripotent stem cell (PSC)-derived cardiomyocytes (CM) -PSC-CM, Poly (D, L-lactic acid)-PDLLA,Poly (D-lactic acid)-PDLA, Poly (Ethylene Glycol)-PEG, Poly (Glycolic Acid)-PGA, Poly (Lactic Acid)-PLA, Poly (Lactic-co-Glycolic Acid)-PLGA, Poly (L-lactic acid) –PLLA, Polyurethane/ laminin-1-PUR/LN1, Polyurethanes-PUR, Vascular endothelial growth factor-VEGF
Introduction
Heart diseases including myocardial infarction result in heart failure which is the leading cause of death worldwide. The prognosis for heart failure is quite poor,and the only solution is organ transplantation, and the situation thereby represents an obstacle because of the lack of donors after their death, or because of the immune response against the graft transplantation. Myocardial infarction represents around 50 % of cardiovascular heart disease. It causes a high level of morbidity and mortality and sequentially ends with heart failure and death. The mammalian cardiomyocytes have a very limited regenerative capacity after their loss, unlike others like a hydra, salamander, and zebrafish,also unlike other organs like skin, and liver, causing permanent and severe heart diseases and the situation thereby heart diseases are the leading cause of death worldwide [1].
Minimal invasive techniques and procedures are extensively required for patients suffering from myocardial infarction or heart diseases generally. The operation has a high risk and requires chest open surgery which exposes many patients to death. Accordingly, some less invasive methods emerged such as a catheter, an endoscope, and needles.As a consequence, scientists are working intensively to uncover and develop new methods and techniques for delivering drugs or enhancing the regeneration ability of cardiomyocytes while using a less intrusive process such as injection.Understanding and mimicking the mechanisms whereby some vertebrates, such as zebrafish or neonatal mammals, regenerate their cardiomyocytes, would provide new insights into the establishment of new strategies to treat heart diseases. Therefore, scientists are grappling with time with much effort in order to discover the mechanisms behind the regeneration or to develop scaffolds to help in the cardiac self-renewal [1,2]. The performed cardiac tissue engineering strategies to determine and achieve cardiac regeneration are either cell therapy using stem cells, bioactive molecules, or tissue engineering using scaffolds made from biomaterial and nanomaterials.
Stem cells introduced a new avenue in terms of cardiac regeneration. They are used to replace damaged and necrotic cardiomyocytes or they enable the proliferation of already existing cardiomyocytes [3]. Different kinds of stem cells tend to help in this regard including induced pluripotent stem cells (iPSCs), mesenchymal stromal cells (MSCs),pluripotent stem cells (PSCs), and embryonic stem cells (ESCs). Interestingly, iPSCs and ESCs can give all cell types and iPSCs can even be derived from normal somatic cells which, in turn, enable autologous engraftment and decrease the possibility of immune rejection. PSCs displayed a higher propensity for angiogenesis, vascularization, and decreased myocardial fibrosis [3]. Moreover, mesenchymal stromal cells have long contributed to cardiac regeneration by induction the cardiac stem cells to proliferate and differentiate into cardiomyocytes or by enhancing angiogenesis [4]. Nevertheless,cell-based therapies have some limitations including the lack of cell retention, a high number of cells to be injected, and immunogenic response in case the cells’ source is allogeneic or xenogeneic [1,5].
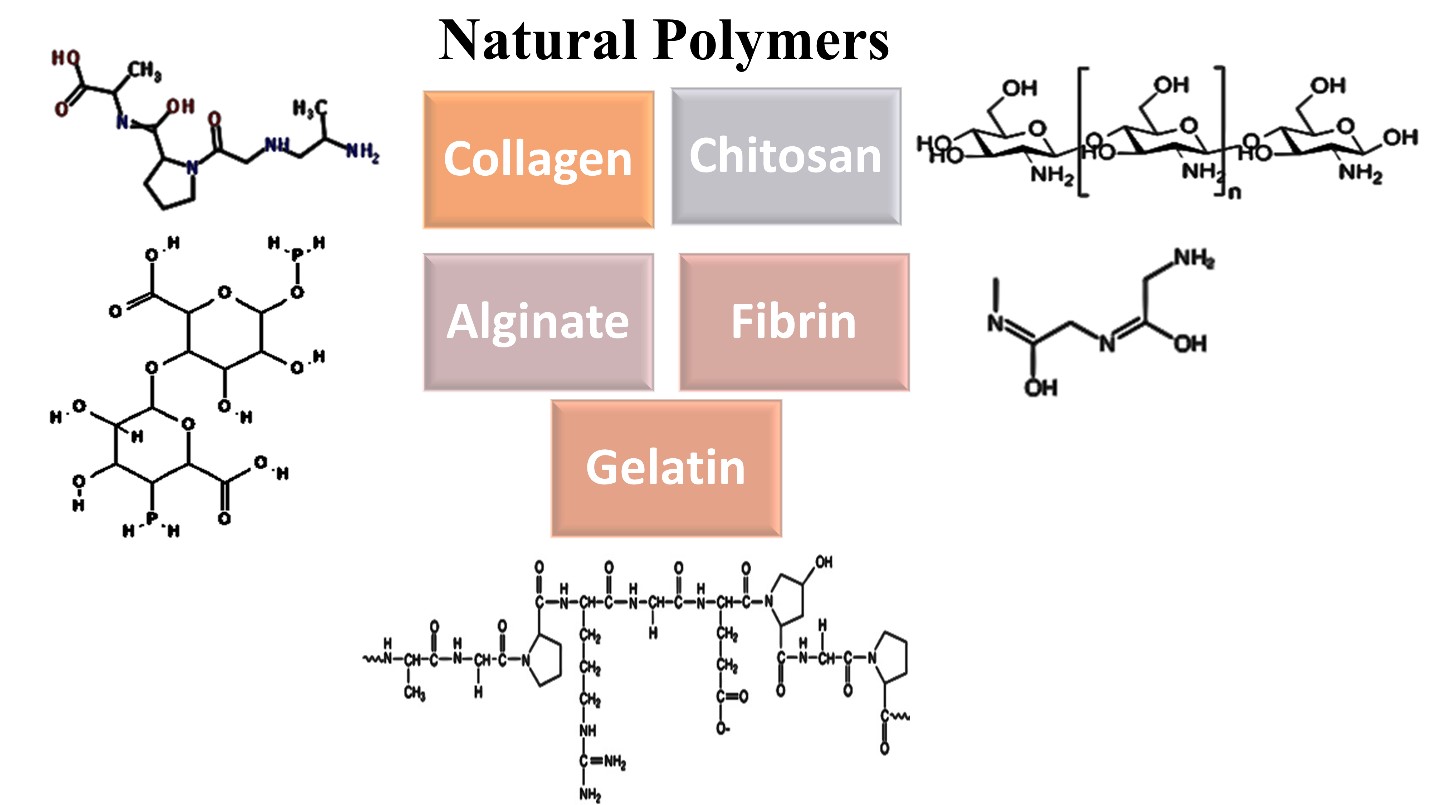
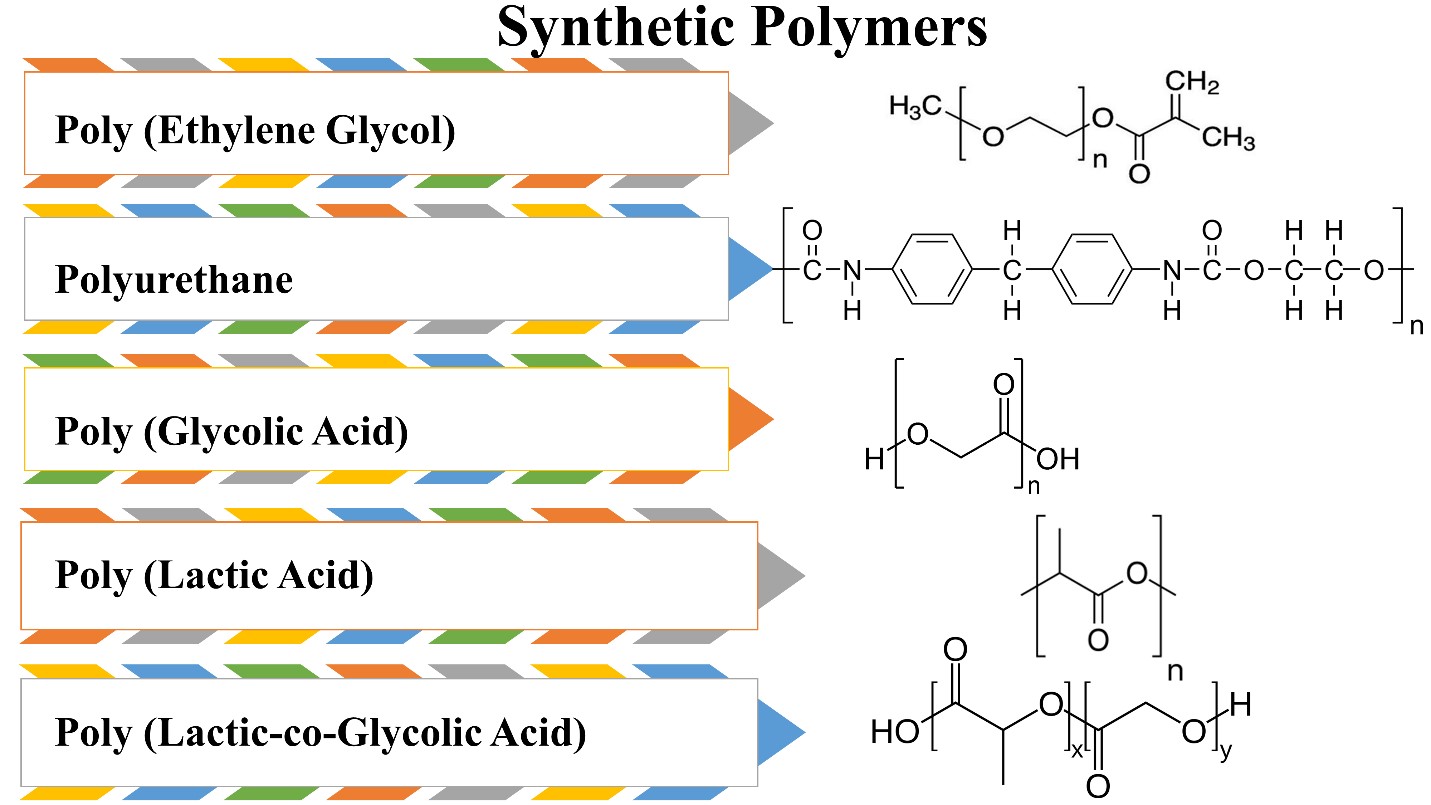
Biomaterials particularly biodegradable polymers are considered valuable tools to deliver the cells, and some exhibit a role in cell adhesion and survival. Several designs or cardiac tissue engineering have emerged including scaffold development as designing a 3D porous structure that mimics the natural extracellular matrix (ECM). Also, hydrogels which can be injected as a liquid form then become gelatinous after crosslinking [6]. Therefore, constructing and seeding a scaffold or hydrogel, alone or in combination with stem cells showed more significant values as they overcome some limitations the cell therapies have, the significance resides in the enhancement of the delivery process and increasing the cell retention level. They are able to recruit cells to the site of injury and induce cell adhesion. Natural and synthetic biodegradable polymers participate significantly in the fabrication of cardiac scaffolds, hydrogels, or patches. They might be used independently, or they can be combined to form a composite. Natural polymers such as chitosan, alginate, gelatin, collagen, and fibrin or synthetic as poly (Glycolic Acid) or (PGA), poly (Lactic Acid) or (PLA), and poly (Lactic-co-Glycolic Acid) (PLGA) exhibited better cardiac regenerative capability [7]. In the present review, we will provide a comprehensive overview of cardiac regeneration approaches, the usage of biodegradable polymers in cardiac tissue regeneration with their pros and cons and we will have insights on recent advances in cardiac regeneration and tissue engineering.
Cardiac Regenerative Capacities and Limitations
The nervous and the cardiovascular systems are considered the systems that do not have the ability to self-renew and are a cause of severe diseases or disability and eventually death. Adult mammals including humans are incapable of heart regeneration; consequently, injury or disease leads to life-threatening heart diseases and death. The failure of cardiomyocytes to divide and proliferate is one of the most obvious hurdles to heart regeneration. On the other hand, some vertebrates like zebrafish, show robust heart regeneration capacity. After cardiomyocyte loss due to injury or disease, the heart becomes incapable of adequate regeneration instead, the heart forms scar tissue which in turn becomes a burden for the contractile activity of the cardiomyocytes.Surprisingly, the concept of the mammalian incapability of heart regeneration, division, and proliferation has been changed. It has been illustrated that the human heart has a modest regeneration capacity with a frequency of 1-22% via cardiomyocyte division. Notably, mammalian neonates can regenerate their cardiomyocytes and compensate for any produced loss Which in turn opened new avenues in heart regeneration induction as a trial to overcome myocardial infarction and heart failure [8-11]
The propensity for heart repair and regeneration in different vertebrate species has been studied extensively to uncover the mysterious limitations of the human heart. Interestingly, newts and zebrafish can regenerate and replace damaged cardiac tissues via the replacement of pre-existing cells. Zebrafish have been used widely in research as a model to understand the mechanism underlying cardiac regeneration owing to their outstanding capability of regeneration.Within two months, it can entirely restore a 20 % heart amputation [12-14]. On the contrary, mice's cardiac regenerative capacity is limited. Cardiomyocyte regeneration develops at a modest annual rate of 0.76 % in mice via the proliferation of pre-existing cardiomyocytes [10]. While neonates exhibited a significant regenerative capacity as a response to injury. Nevertheless, this regenerative capacity diminishes over time, as within a week mice become unable to regenerate their hearts one more time [11]. They have the same regenerative mechanism as zebrafish which is re-inserting the cardiomyocytes into the cell cycle [15,16]. Previously, it was thought that humans do not have any cardiac regenerative capacity. Surprisingly, rejuvenation of human cardiac cells has been shown to continue during human life even if at a modest rate estimated at 2% in the first decade of life and declined to 1% in the 7th decade [9].
The underlying mechanisms that govern cardiac regenerative limitations are not fully understood. Determination of different regeneration among vertebrate species will provide insights into the limitation of other species and most importantly human beings. Intrinsic variations in cardiomyocytes could be involved in this. In comparison to mature mammals, lower vertebrate cardiomyocytes are mononucleated, smaller in size, and contain fewer myofibrils.These features have been found in young mammals suggesting that they may induce regeneration via cell cycle re-entry. Therefore, these variations might be attributed toenhancing or suppressing cardiac regeneration [17].
Although cardiomyocytes play a substantial role in cardiac regeneration potential., non-cardiac cells have been found to be attributed to poor regenerative capacity when they respond to injury or disease. For instance, fibroblasts are abundant in adult mammalian cardiac cells unlike the fetal mammalian nor the adult non-mammalian vertebrate.They have an inverse effect on cardiac regeneration, and they are contributed to scar tissue formation. Anatomically, the Zebrafish heart has a different trabeculae structure than the adult mammalian heart but is similar to the fetal mammalian heart. In zebrafish, it is long, extensively lined with endothelial cells and it protrudes into the ventricular lumen. In contrast, in the adult mammalian, the wall of the ventricle becomes thicker with less trabeculation and endocardial surface area, suggesting that this is involved in the regeneration potentiality [17].
Possible Cardiac Tissue Regeneration Approaches
Stem Cell-Based Therapy
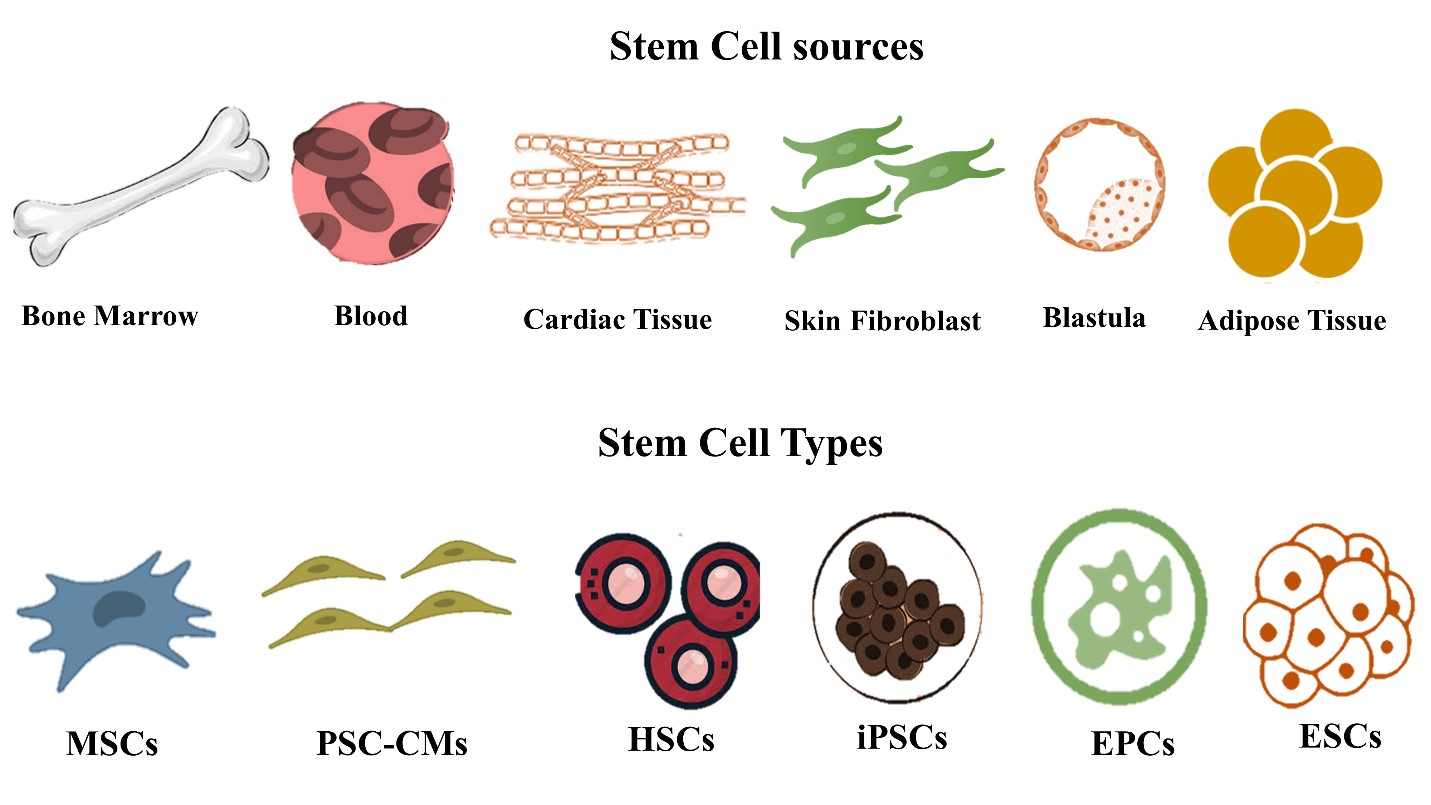
Cell-based therapy and particularly stem cell-based therapy participated dramatically in the treatment of urgent cardiovascular disorders as a myocardial repair that leads to heart failure. Huge efforts have been made to test a variety of cell types for the purpose of heart regeneration.For instance, embryonic stem cells, mesenchymal stromal cells, hematopoietic stem cells (HSCs), induced pluripotent stem cells, pluripotent stem cell (PSC)-derived cardiomyocytes (CM) (PSC-CMs) and endothelial progenitor cells (EPCs) as illustrated in figure 1 [1,5,18-23].
The cell sources might be autologous, allogeneic,or xenogeneic. The autologous source is the source of choice because of the absence of immune rejection. Stem cell source varies according to the stem cell type, for instance,stem cells can be extracted from bone marrow, blood,cardiac tissue, the inner cell mass of the blastocyst, or adipose tissue as shown in figure 1. Then these extracted stem cells undergo differentiation into cardiomyocytes, endothelial cells, or smooth muscle cells [1,5,18-23].
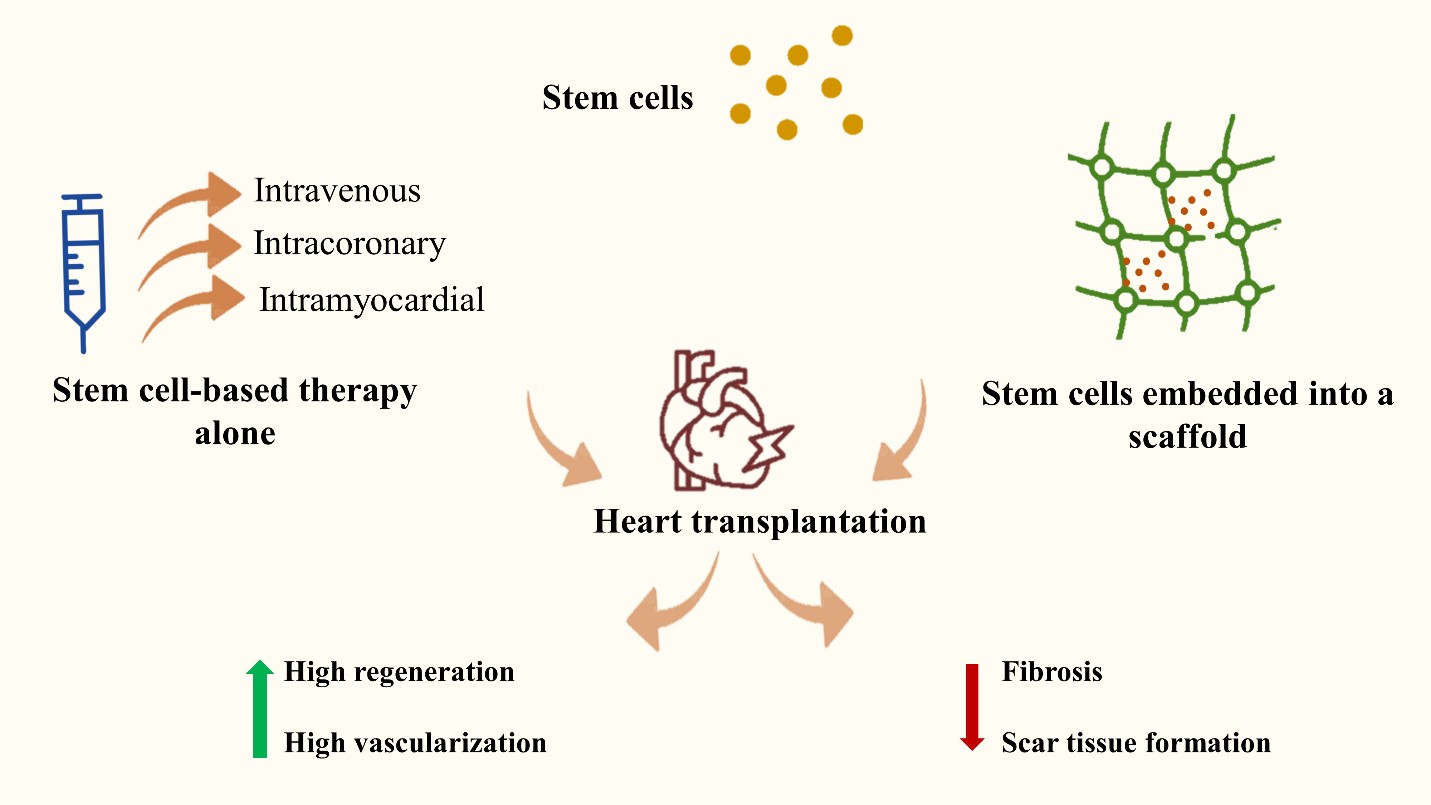
The stem cells could be administrated intravenously,intracoronary, or into the myocardium directly in a form of cell suspension. On the other hand, their delivery could be performed with the help of other supporters such as sheets, scaffolds, or others. They could be administrated with or without growth factors or exosomes that enhance the process of proliferation, adhesion, and attachment.When administrated, they exhibited high regeneration, and vascularization and they decreased fibrosis and scar tissue formation (figure 2) [1,5].
Cell-based therapy showed promising results in the process of regeneration, still, there are problems in cell retention and a high number of cells to be administrated. Induced pluripotent stem cells are a new type of stem cell that can differentiate into all cell lineages and are easily obtained by the conversion of somatic cells into iPSCs [22]. While MSCs are a heterogeneous population of stem cells that exist in the vast majority of stromal tissues. Their heterogeneity allows them to be multipotent stem cells, or progenitors, or even differentiated cells [24,25]. The ability of MSCs to self-renew and undergo multidirectional differentiation including cardiomyocytes is therefore only demonstrated by a small portion of the population which are the multipotent stem cells and progenitors [24,25].
Biomaterials are considered a promising method too in the process of regeneration, they act as a good scaffold,and vehicle, and play a substantial role in stem cell retention and survival, they also can be combined with specialized cells [22].
Decellularized Extracellular Matrix (dECM)
Another aspect that can be used in cardiac tissue regeneration is the decellularized extracellular matrix (dECM) which is extracted from natural cardiac tissues (myocardium).It represents a natural scaffold and template but without cellular elements. It has been widely studied as a natural alternative to cardiac regeneration in the myocardial heart. It maintains the native microenvironment and the microstructure.It provides native mechanical stability and retention of cellular elements and biomolecules and consequently enhances recellularization through proliferation and differentiation.
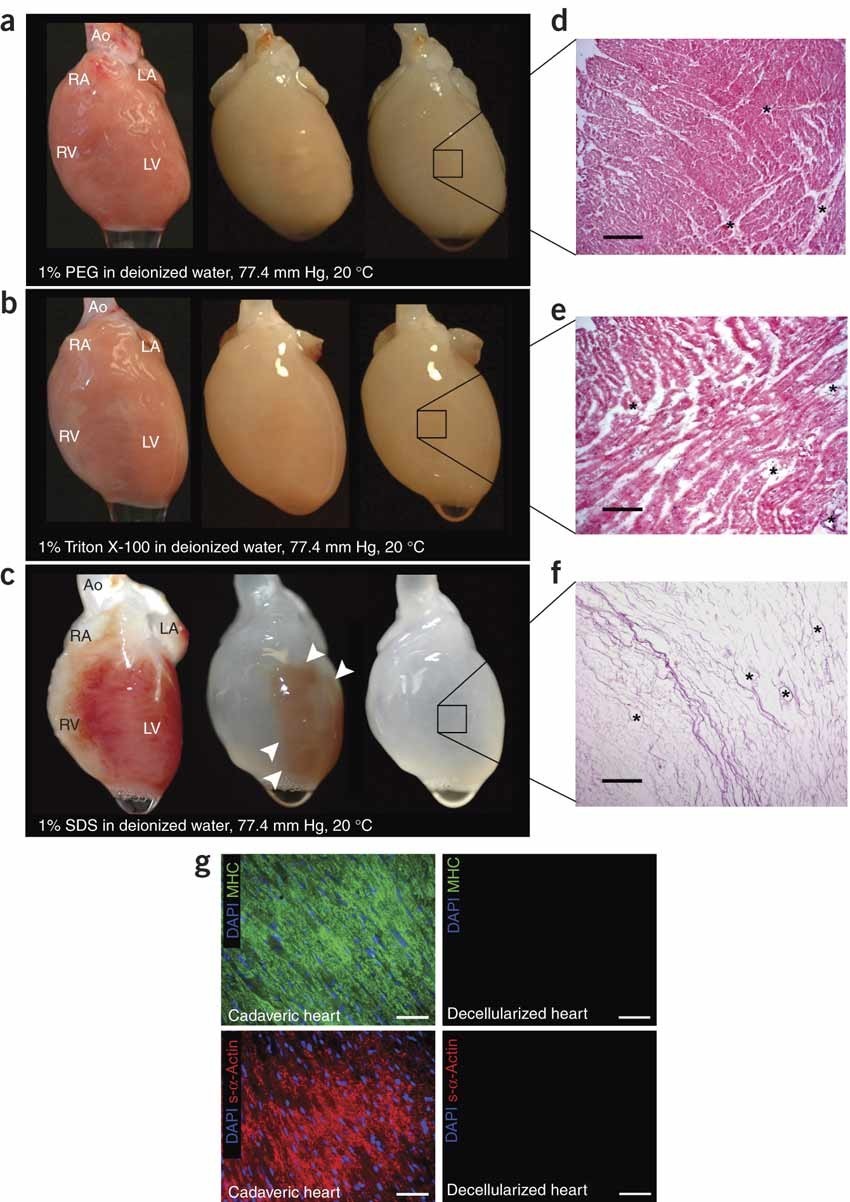
The decellularization strategies and approaches vary. They include three major categories; 1) The chemical approach that depends on using a chemical compound to remove the intact cells and keep only the matrix. It uses acid,detergents, bases, or alcohol. 2) The enzymatic or biological approach in which biological compounds are used to achieve cell removal. For instance, enzymes such as trypsin.Also, chelating agents can be used as biological materials for decellularization. 3) physical approach, this approach uses physical forces like pressure or mechanical forces, and electroporation to extract cells. On the other hand, freezing and thawing are also used [26].
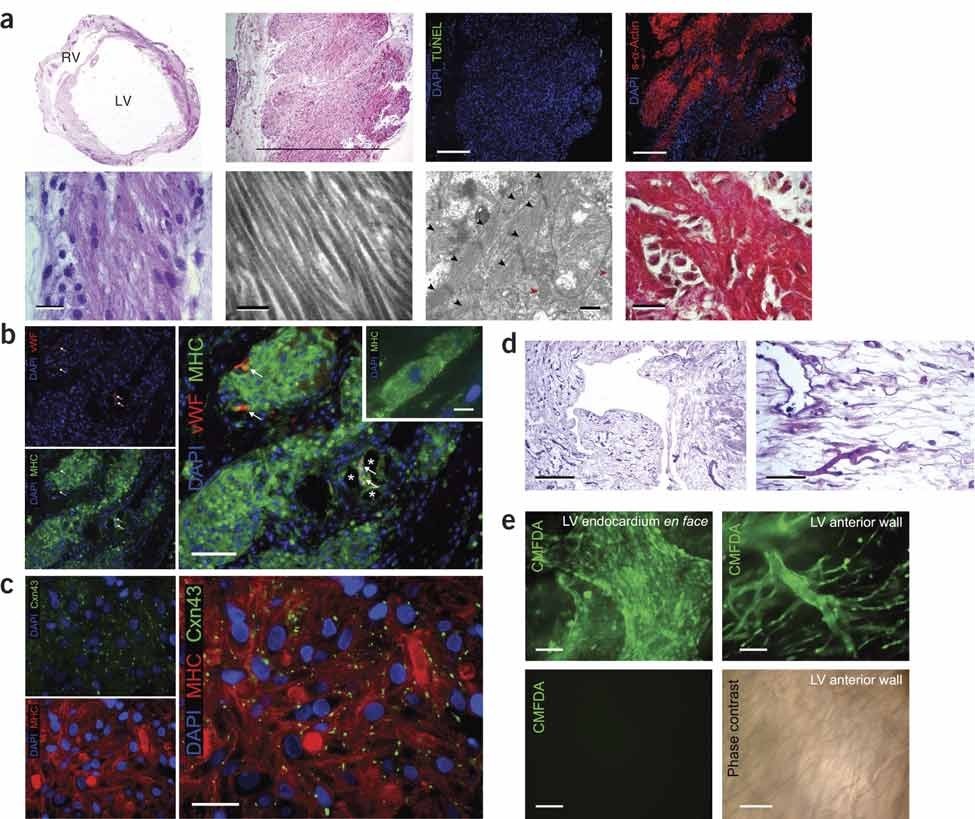
ECM whole heart was performed on rats and porcine, and it managed to maintain the ECM and the whole heart architecture. Interestingly, the recellularization took place significantly when cardiac and endothelial cells are seeded to the ECM [27,28]. Because of these significant results and the advantages, the ECM displayed, scientists moved to try this approach in humans. Sanchez et al. developed the first ECM for the human heart which was found to keep the heart shape, vascularity, and mechanical stability.They achieved recellularization, regeneration, and vascularization after cell reseeding [29]. These results indicated that ECM represents a suitable natural platform for cardiac tissue regeneration. Nevertheless, there are still major obstacles that exist when using dECM, including adequate recellularization to achieve the goal. Also, the balance between ECM preservation and cell removal is illustrated in figures 3 and 4.
Scaffold-Based Therapy Via Tissue Engineering
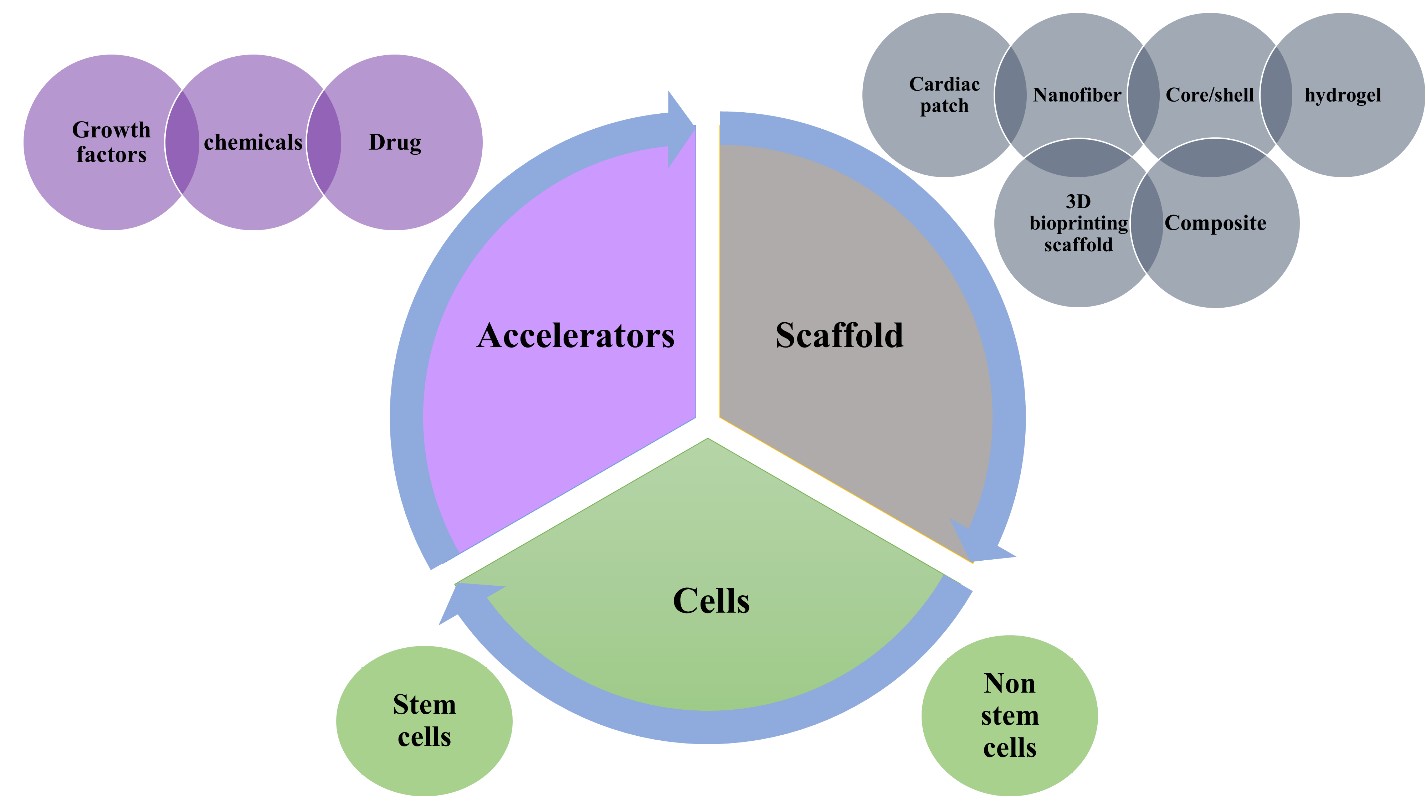
Cardiac tissue engineering presents the problem of developing and regenerating damaged heart valves and myocardial muscles. It provides a revolutionary breakthrough in cardiac regeneration. It provided solutions for cardiac repair, healing, proliferation, differentiation, and consequently regeneration. In tissue engineering, it depends on the fabrication of scaffolds using biomaterials as polymers to represent a template and support for the heart to regenerate the damaged part. It mostly occurs with the help of cellular elements such as stem cells or non-stem cells and/or bioactive molecules as growth factors (fig.5). The process involves isolating cells from a patient via biopsy and then culturing them in a scaffold that resembles the original tissue environment.The scaffold should possess specific features that allow tissue regeneration and replacement, it will be discussed in the following section. The selected biomaterials must act as biological and mechanical support, it should enhance cell adhesion, growth, and proliferation. Different fabrication techniques are used in order to design scaffolds ranging from electrospinning, and 3D bioprinting which has been widely used. Different scaffold designs have been shown as cardiac patches, nanofibers, core/shell, and hydrogels as illustrated in figure 5 [6,30].
Characteristics of Scaffolds and Biodegradable Polymers
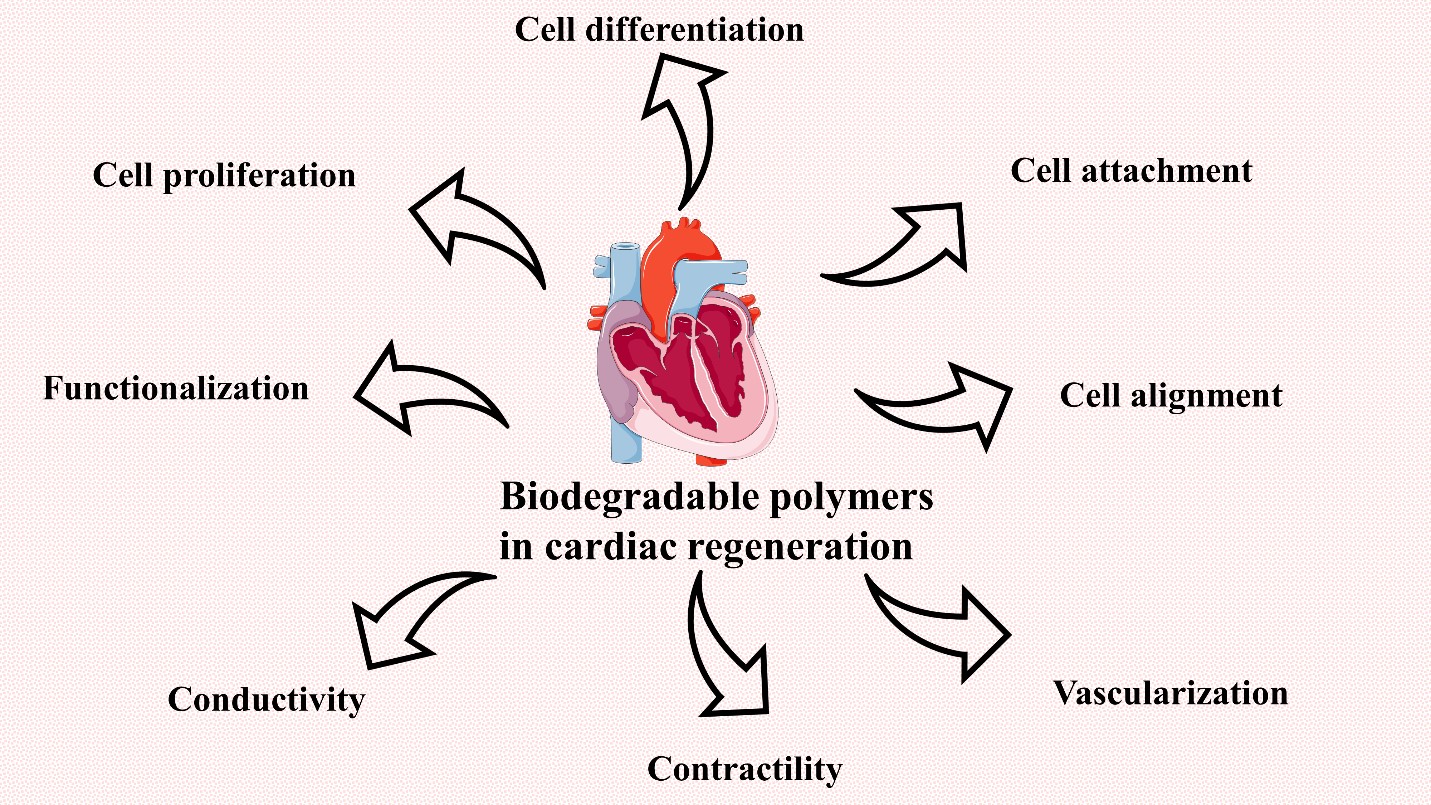
Tissue engineering has made substantial use of biodegradable polymers. Polymer types, properties, and physicochemical qualities are all significant factors to consider while designing a scaffold that enhances tissue growth.To design a scaffold able to maintain heart function and regenerate the damaged cells, the scaffold should have particular criteria to achieve the best regeneration and healing capability.The scaffold should mimic the extracellular matrix present in the natural heart. It also should serve as a repository for the slow release of bioactive chemicals, and allow nutrients, oxygen, and waste to pass from and out of the cardiac tissue. It needs to allow angiogenesis and vascularization;therefore, it should be porous enough to allow vascularization.Importantly, it should be mechanically stable and strong to endure the harsh environmental condition and the surrounding contraction. It also must resemble the native cardiac tissue. Another necessary feature needed to present in the scaffold is to be conductive to serve as a biological pacemaker and cell support, it also affects the cell behavior.Biocompatibility and biodegradability are substantial features required in the ideal scaffold. A scaffold must not cause coagulation or immune response, it also needed to be degraded gradually upon regeneration or proliferation.It should remain for a long enough time to allow cells to be integrated with the native tissue. When degraded, it must not secrete any toxic substance. Biodegradable polymeric biomaterials have a great influence on cardiac tissue engineering. They have a suitable rate of biodegradation that is long enough to allow cells to adhere and proliferate and at the same time not to remain for a longer time than needed to avoid allergic reactions or safety concerns. Several natural and synthetic polymers serve as good biodegradable sources with proper physical, chemical, and biological properties. The used polymeric materials should allow cell adhesion, proliferation, alignment, and differentiation.They also should permit the occurrence of vascularization and angiogenesis. Moreover, they should be electroconductive which is the major feature of cardiac tissue that allows action potential passage (figure 6) [31-33].
Then the designed scaffold can be seeded with cellular elements. Cell source differs from autologous, allogeneic,or xenogeneic sources. Cell type ranges from stem cells (embryonic stem cells, nuclear transfer embryonic stem cells, induced pluripotent stem cells, mesenchymal stromal cells, cardiac stem cells, purified hematopoietic stem cells,and many others) or skeletal myoblasts or progenitor cells or bone marrow mononuclear cells [1,32,34]. Cell-based therapy alone has limitations including low cell retention, targeting, and delivery. Therefore, using biomaterials and scaffolds aids in the improvement and overcoming of such obstacles. They act as a support and a vehicle to maintain cells and deliver them to the target tissue. They also support the cells mechanically to help in proliferation and regeneration with keeping the same tissue architecture [32].
Growth factors play a substantial role in cardiac tissue regeneration as they are able to enhance the process of angiogenesis. For instance, in coronary artery disease,which is implicated by an arterial obstruction, the process of angiogenesis becomes necessary for cardiac repair and function maintenance. This occurs by growth factors that cause the formation of new blood vessels. On the other hand, growth factors enhance cell adhesion, migration, proliferation,and differentiation which in turn, promotes regeneration and cardiac repair. They cause ECM remodeling,and homing of stem cells or proliferation of cardiomyocytes, they have an antiapoptotic effect. Interestingly, injected stem cells in the damaged heart tissue can regenerate it via recruitment and secretion of growth factors indicating the involvement of these molecules in cardiac regeneration and repair. Unfortunately, growth factors have a short halflife with declined stability. Therefore, they are integrated into scaffolds and/or mixed with stem cells [35].
Classification of Biodegradable Polymers used in Cardiac Tissue Regeneration
Natural Biodegradable Polymers in Cardiac Scaffolds
Collagen
An essential natural polymer that is widely used in the field of tissue engineering in general and in cardiac tissue regeneration, in particular, is collagen. It is the most prevalent protein in the human body, it can be found in skin,cartilage, tendons, and bone. Moreover, it provides structural support to other tissues, such as blood vessels. It consists of polypeptide strands that are responsible for producing collagen fibers with suitable mechanical properties [36,37].
Collagen comes in a variety of forms; there are approximately 28 different types. Types I, II, III, and IV are,nonetheless, commonly utilized in tissue engineering. Heat feasibility, biodegradability, hyposensitivity, biocompatibility,low toxicity, and robust cellular activity are all advantages of collagen for cardiac tissue engineering. Collagen,on the other hand, exhibits inferior mechanical and electrical properties. Furthermore, when hydrated, it loses structural stability [7,38-44].
To compensate for the lack of mechanical stability,collagen scaffolds are physically or chemically crosslinked to produce stiffness-controlled collagen scaffolds. In a study done by Lin et al, collagen type I was cross-linked with different ratios of hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDC). Then these scaffolds are seeded with mesenchymal stromal cells. The results revealed that this scaffold enhanced mesenchymal stromal cell proliferation and differentiation into cardiac progenitor cells [45]. Collagen combined with carbon nanotubes (CNTs) is another solution to circumvent collagen's weak mechanical properties. Carbon nanotubes were chosen because of their outstanding mechanical and electrical capabilities. By taking into consideration the toxic high dose of carbon nanotubes, combining collagen and carbon nanotubes boosted electrical conductivity, cell alignment, and assembly [46].
Another study used varying ratios of collagen type I and carbon nanotubes to construct a scaffold, which was then seeded with LX-2 cells and cardiomyocytes separately.The findings demonstrated that LX-2 cells implanted on collagen carbon nanotube scaffolds did not undergo apoptosis or alteration in cell morphology. On the other hand, when the scaffold was seeded with cardiomyocytes, cell function was improved. Carbon nanotubes increased the scaffold's electrical and mechanical properties [47]. Nanofibrous collagen scaffolds are manufactured by electrospinning, in which collagen is combined with glycerol and compatible solvents before being seeded with the cellular element which is induced pluripotent stem cell-derived cardiomyocytes. In dilated cardiomyopathy, these scaffolds containing cellular components exhibited promising results [48].
Gelatin
Gelatin is a natural polymer derived from collagen that is employed in cardiac regeneration because of its biodegradability, biocompatibility, safety, and nontoxicity in addition to its limited immunogenicity. The fabrication of a high stable scaffold in the form of gelatin matrices by electrospinning has been demonstrated to promote cardiac cell development. These nanofibrous matrices possessed biophysical and mechanical characteristics that were ideal for contracting cardiomyocytes. On the other hand, gelatin was found to provide a weak mechanically stable scaffold with poor suture retention. Furthermore, it is extremely hydrated and has a short shelf life. Therefore, cross-linking of gelatin chemically, physically, or biologically via enzymes has been used to overcome gelatin disadvantages and produce an appropriate scaffold. For instance, when gelatin was cross-linked using microbial transglutaminase enzyme to obtain gelatin/microbial transglutaminase hydrogel, it enhanced cell proliferation, differentiation, and adhesion. Furthermore,this cross-linked scaffold was nontoxic with controlled the mechanical properties of the hydrogel [7,49-51].
Fibrin
Another natural polymer, which is a cross-linked biopolymer, that is extensively used in cardiac regeneration and tissue engineering is fibrin. It is consisting of fibronectin,and it is involved in blood clot formation, in presence of thrombin which converts fibrinogen into fibrin with clot formation. The produced clot can be dissolved in presence of other enzymes [52]. This is because it is safe, non--toxic, injectable, high biocompatible, biodegradable, and has a controllable degradation rate, it enhances cell proliferation,adhesion, and attachment. Its gelation rate is depending on the modulation of fibrinogen and thrombin ratio However, it has some drawbacks as weak mechanical stability and susceptibility to gel shrinking. Furthermore, it can transmit diseases. In order to overcome these drawbacks fibrin was mixed with synthetic polymers to enhance elasticity and resistance to deformation forces [7].
Fibrin has been used to selectively isolate cardiac stem cells through recapitulation of heart homeostasis when it is used in designing a 3D cardiac culture system. The selective isolation of cardiac stem cells is carried out via fibrinolysis of the scaffold and matrix without further processing as tissue purification or dissociation [53].
A commercial fibrin glue product, approved by the FDA and has been used in surgery is called Evicil® (Ethicon) which has been. It has been used as a tissue sealant and hemostatic agent. The manufacturing depends on the thrombin enzyme will make cross-linking to the fibronectin into a clot that rapidly closes the wound. The property of cross-linking allowed fibrin to be widely used in drug delivery,and cell cargo. Moreover, it can be facilitating its modification and combination [32]. On the other hand, fibrin has been used as a source for cardiac angiogenesis. Fibrin stimulated cardiac angiogenesis via two main growth factors: vascular endothelial growth factor (VEGF) and platelet-derived growth factor-BB (PDGF-BB). This result has been obtained without any genetic modification or transfection suggesting that fibrin plays a crucial role in inducing cardiac angiogenesis without any side effects [54].
Alginate
Alginate is a natural anionic polysaccharide that is commonly used in cardiac regeneration. It consists of β-D--mannuronic acid and α-L-guluronic acid. It can be obtained from the cell walls of brown algae like Ascophyllum nodosum,Laminaria japonica, Laminaria hyperborea, and Sargassum.Alginate has different biochemical properties that make it a good choice in biomedical applications such as high biocompatibility, biodegradability, easy graft co-polymerization,non-toxicity, low cost, and non-thrombogenic.Furthermore, it can cause improvement in the biological features and capability of mechanical property modification.However, gel degradation is still one of its limitations that causes refraining from using it in a variety of applications.However, this drawback can be defeated in vivo, when alginate is oxidized with periodate or irradiated with gamma radiation.Moreover, high hydrophilicity is another disadvantage that limits cell attachment and proliferation. It has weak mechanical stability as well which can be overcome when combined or copolymerized with other degradable polymers that have high mechanical stability [7,30,55]. Alginate has been combined with fullerenol to form fullerenol/alginate hydrogel and injected into a myocardial infarction rat model. It has been used as cell delivery in cardiac tissue regeneration. It exhibited antioxidant activity with no cytotoxicity on the used stem cells. It enhanced stem cell delivery, adhesion, and survival. This scaffold enhanced stem cell differentiation to cardiomyogenic [56].
Chitosan
Chitosan is a natural polymer derived from chitin in marine crustaceans and is produced from β-(1-4)-D glucosamine and N-acetyl-D-glucosamine. It is commonly used in tissue engineering including cardiac tissue engineering because of its flexibility to be fabricated using different fabrication techniques to give different forms such as nanofibers,gel, patches, or 3D scaffolds. Scaffolds made by chitosan exhibit high safety, high biocompatibility, and biodegradability. Chitosan exhibits antibacterial characteristics and high wound healing properties with no immunogenic response. Chitosan hydrogel possessed antioxidant activity and cell adhesion with a decrease in oxidative stress in cardiac tissue engineering [30]. Chitosan is mechanically weak, but with cross-linking with polymers, the mechanical stability is improved. For instance, it is combined with natural polymers as collagen or alginate or with synthetic polymers as poly (Lactic Acid), poly (Lactic-co-Glycolic Acid),and poly (Ethylene Glycol) or (PEG). This produced several forms of scaffolds with higher mechanical stability [7,36].
Chitin is insoluble in several solvents. In contrast,chitosan is created via chitin deacetylation producing soluble chitosan. Chitosan can be degraded with a variable number of enzymes including lysozyme which is the main enzyme that degrades chitosan physiologically in vivo. The degradation rate of chitosan depends on the degree of acetylation.For instance, chitosan which has a low acetylation rate can last for a long period in vivo without degradation.The degradation rate can be controlled via side group modification [7,36]. Chitosan is widely used in cardiac regeneration as its degradation products are biocompatible with a controlled degradation rate. For instance, Xu and colleagues injected chitosan hydrogel to deliver mesenchymal stromal cells. They designed the scaffold to be temperature-responsive,this scaffold revealed that it enhanced cell retention,and improved mesenchymal stromal cell differentiation into cardiac cells when administrated to myocardial infarction rat model [57].
Matrigel
Matrigel is a natural extracellular protein-containing natural polymer including collagen, laminin, entactin,and perlecan. Laminin supplies Matrigel with properties that allow it to function as the basement membrane's extracellular environment. It is extracted from Engel breth-Holm-Swarm (EHS) mouse tumors. It has been used as a gelatinous material in cardiac tissue engineering to enhance cell adhesion and attachment, it also shows a robust effect on angiogenesis. Matrigel contains numerous precious growth factors that facilitate cell proliferation, growth,and differentiation. The presence of a high protein content improves matrix stiffness and scaffold strength. It has been manufactured commercially by extracting it from mice tumors and it has a long shelf life (2 years). The obstacle that prevents its use in clinical trials or as a product in the market is that it has an animal source as it is derived from the sarcoma cells of mice [7]. A study used Matrigel to produce vascularized pacemakers using endothelial and cardiac progenitor cells. These cells were seeded and incorporated with the Matrigel and transplanted in vivo in a rat model and investigated in vitro as well to detect and examine alterations in the cardiac sinus node. The results illustrated that this scaffold elevated the survival rate in rats and scaffold improved the electrical activity indicating that the tissue-engineered Matrigel scaffold seeded with endothelial and cardiac progenitor cells acts as a cardiac pacemaker. However,this scaffold didn’t achieve effective vascularized tissue [58].
Synthetic Biodegradable Functional Polymers for Cardiac Tissue Engineering
Poly (Ethylene Glycol) (PEG)
Poly (ethylene glycol) is a biodegradable synthetic polymer, derived from ethylene oxide. It is used in cardiac tissue regeneration because of its high safety, and high solubility as it is soluble in water and organic solvents, exhibits preferable mechanical properties and is easy to manipulate the chemical composition when entered into hydrogel design or scaffold. Nevertheless, the limitations in using it as a polymer in tissue engineering are less cell adhesion capability,it is an inert polymer so it will decrease cell proliferation and differentiation [7]. PEG has been used in a form of composite as well in cardiac tissue regeneration. For instance, it has been combined with fibrinogen to form a PEG-fibrinogen patch which was used as a supportive scaffold with genetically engineered induced pluripotent stem cells expressing placental growth factor (PlGF) and matrix metalloproteinase 9 (MMP9). This scaffold aided in repairing ischemic and damaged myocardium, it enhanced cell proliferation, growth, and differentiation [59]. PEG has been used also in a form of hydrogel when it is mixed with heparin and seeded with mesenchymal stromal cells. This scaffold enhanced cardiac cell retention, cell growth, and angiogenesis [60].
Polyurethane (PUR)
Polyurethanes have been used in biomedical applications as tracheal tubes. Also, they are used in cardiac regeneration and cardiac assist devices [61]. They have been synthesized by condensation of diisocyanates with both alcohols and amines. They are considered biocompatible, and mechanically stable strong polymers. They have a degradation rate that resembles polyesters, pure polyurethanes have a very low degradation rate, and this makes them poor candidates for drug delivery and tissue engineering applications. Nevertheless, polyurethanes enhance cell proliferation and adhesion and have good mechanical stability.Therefore, using them in combination is a better way to exploit their advantages and overcome their disadvantages [36].
polyethylene glycol-based polyurethanes manufactured with poly(epsilon-caprolactone) have been fabricated in a form of a porous patch and they enhanced cell adhesion and proliferation [62,63]. Moreover, they used a Lewis rat model in a form of bi-layered patches from polyurethanes and extracellular matrix, this patch induced angiogenesis and inhibited scar formation [64].
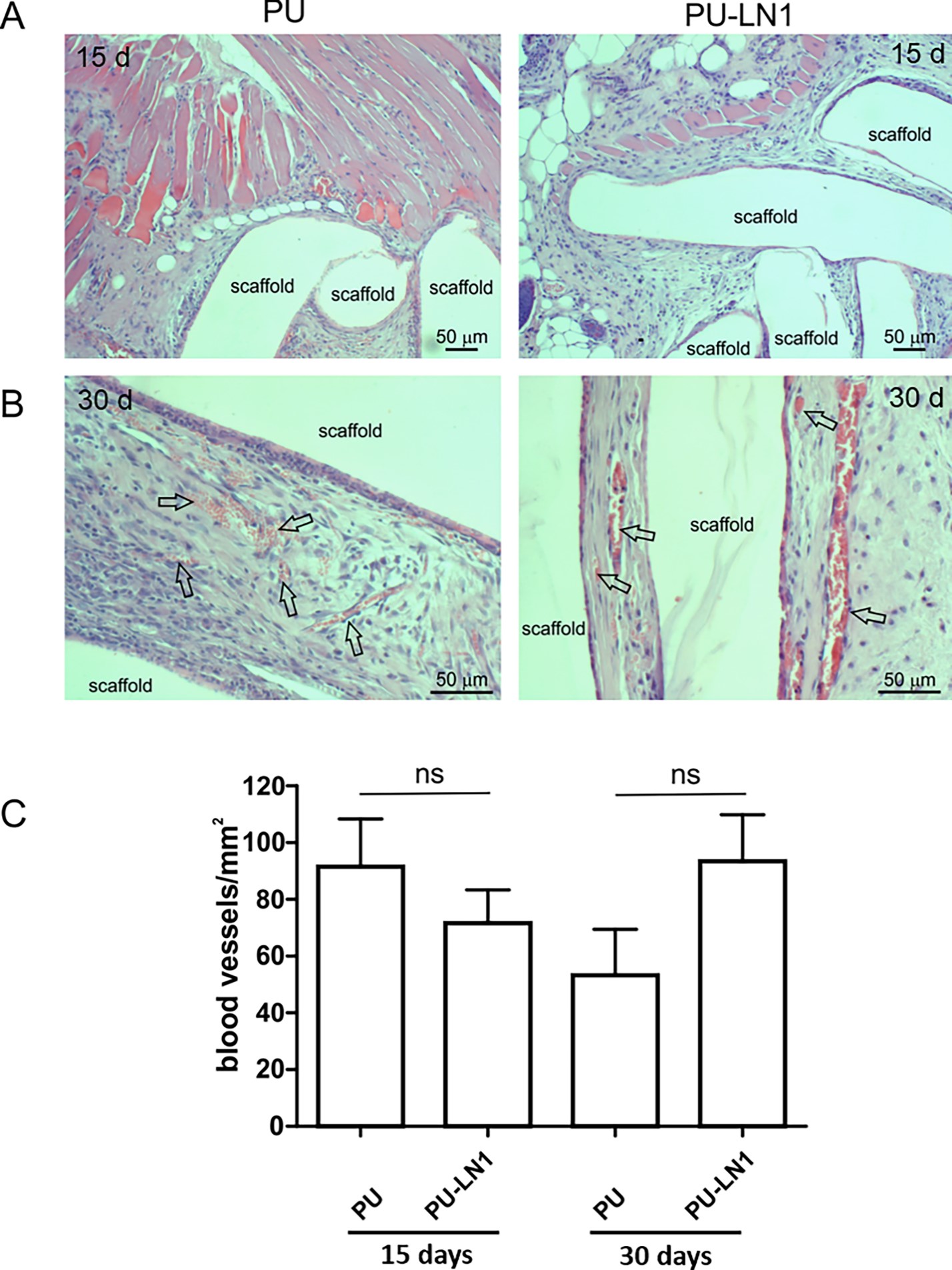
Polyurethane/ laminin-1 (PUR-LN1) mimicked the microenvironment of the cardiac tissue. This scaffold has been fabricated by surface functionalization and modification of polyurethane via melt extrusion/ carbodiimide chemistry. Then they are grafted with laminin-1 (LN-1) which is a cardiac extracellular matrix. PUR has been also combined with gelatin which acts as an adhesion molecule.Both scaffolds performed better cell adhesion. PUR-LN1 induced cell proliferation and differentiation with protection from apoptosis [65]. Additionally, a subcutaneous administration of PU-LN1 scaffold in mice was efficiently absorbed into the host tissues, with no evidence of resorption or significant inflammation (figure 8).
Using the PUR-LN1 scaffold without cell seeding was found to induce and facilitate long-term angiogenesis as illustrated in figure 9 [65].
On the other hand, a network of polyurethane and siloxane has been fabricated for cardiac regeneration applications.The scaffold was combined with an electroconductive source which was aniline tetramer (AT), a biodegradable source which was castor oil, and a source for mechanical stability which was siloxane. This network achieved better C2C12 myoblasts adhesion and proliferation [66].
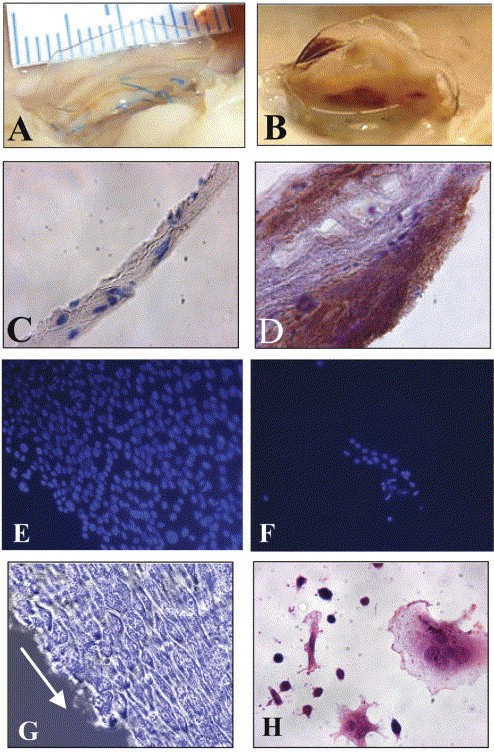
PURs showed their efficiency when applied in heart valve development. Previously, when heart valves made of PURs were applied, calcification originated after blood contact, which later resulted in thromboembolic activity [67]. Therefore, to overcome the calcification and develop an anti-thrombogenic surface in addition to boosting cell attachment, Stachelek and colleagues developed a valve made of polyether urethane. They used cholesterol-modified PUR (PUR-Chol) to stimulate endothelial cell proliferation,and adhesion [68]. Increased surface energy, a reasonably smooth surface, and distinct surface chemistry distinguished PUR-Chol from untreated PUR. This valve enhanced the seeding, adhesion, and retention of the blood outgrowth endothelial cells (BOECs) with no gross abnormalities,unlike the unseeded polyurethane leaflets which exhibited thrombosis as shown in figure 10A and B. The seeded leaflet did not induce any immunogenic reaction upon implantation, unlike the unseeded part as shown in figures 10C and D [68].
Poly (Glycolic Acid) (PGA)
Poly (Glycolic Acid) is a simple synthetic polymer used extensively in tissue engineering. It is a linear aliphatic polyester that is characterized by a high melting temperature of more than 200 °C, safe degradation products, biocompatibility,mechanical stability, and biodegradability.However, its high hydrophilicity allows PGA to lose its mechanical stability when applied in vivo after 4 weeks of grafting.Therefore, it is used as a temporary scaffold in cardiac regeneration. Surface modification is performed to enhance PGA's capability to enhance cell adhesion and proliferation [7].
Poly (Lactic Acid) (PLA)
Poly (Lactic Acid) is semicrystalline polyester that is obtained via the polymerization of lactic acid. It is biodegradable with a low degradation rate. It is a hydrophobic polymer that is found in different forms; meso-poly (lactic acid), poly (L-lactic acid) (PLLA), poly (D-lactic acid) (PDLA), and finally poly (D, L-lactic acid) (PDLLA). Each form has its features and applications. For instance, regarding crystallinity, PDLA is considered a crystalline material while PLLA is a semicrystalline polymer. On the other hand, meso-poly (lactic acid) is amorphous. PLLA is widely used in heart regeneration because of its safety, biocompatibility,mechanical stability, and biodegradability. However,its main drawback is the low degradation rate [7].
Poly (Lactic-co-Glycolic Acid) (PLGA)
Poly (Lactic-co-Glycolic Acid) is a copolymer of both PGA and PLA. It is suitable for cardiac regeneration because of its mechanical property, biodegradability, biocompatibility,controllable degradation rate, and safety. It is also less soluble in organic solvents. However, its disadvantages are limited cell adhesion, and proliferation [7]. PLGA has been fabricated in a form of a core/shell using electrospraying. It is combined with stromal-derived factor- 1α and seeded with mesenchymal stromal cells applied for the purpose of treating myocardial infarction. This core/shell exhibited a good vehicle for drug delivery as it made sustain release of the incorporated stromal-derived factor- 1α. It also enhanced stem cell proliferation [69].
Advantages and Disadvantages of Natural and Synthetic Biodegradable Polymers
Natural biodegradable polymers exhibit special characteristics that allow them to be widely used in regenerative medicine and heart regeneration. For instance, fibrin,gelatin, and alginate are nontoxic. Collagen has very low toxicity. They also display some disadvantages that become a burden in some circumstances. Fibrin has high elasticity and biocompatibility. It also enhances cell adhesion with control of its degradation rate. While its drawbacks, it may allow disease transmission, and it is not mechanically stable.While collagen is characterized by good permeability, biocompatibility,and biodegradability without stimulation of the immune system when applied. However, mechanical,and electrical properties are extremely weak. Similarly, chitosan exhibited the same advantages as collagen besides its antimicrobial activity and hemostatic property. It is easily fabricated and processed. Nevertheless, it is so fragile, and it is soluble only in an acidic medium which acts as a burden as it makes it less soluble in physiological PH. Gelatin and alginate have outstanding nontoxicity, biocompatibility, and biodegradability. Alginate is a chelating agent with nonthrombogenic nature. While disadvantages for gelatin and alginate are low melting temperature and low protein adsorption, respectively [7].
Synthetic polymers such as poly (Glycolic Acid),poly (L-lactic acid), poly (Lactic-co-Glycolic Acid), poly(Ethylene Glycol), polycaprolactone, and polyurethanes are biocompatible and nontoxic. Poly (Glycolic Acid), and poly (Lactic-co-Glycolic Acid) are biodegradable with high and adjustable degradation rates respectively. While polycaprolactone,poly (L-lactic acid), and polyurethanes have a long degradation time. PLLA and PLGA have good mechanical properties, while PGA is less mechanically stable. PGA and PLGA are low soluble in organic solvents while polyurethanes are soluble in organic solvents. Regarding cell adhesion, growth, and proliferation, PLGA and PEG are not the polymers of choice because they possess low cell adhesion, and proliferation capability [7].
Possible Fabrication Techniques in Cardiac Tissue Regeneration
Various fabrication techniques have been employed for cardiac tissue regeneration purposes and scaffold design. The fabrication technique of choice is determined by the function for which it is built, and the materials used to construct it. The manufacturing method utilized has a significant impact on the scaffold's properties and, as a result, its functionality. Admitting that the major features of the scaffold play a key role in heart regeneration effectiveness, the manufacturing processes undoubtedly have a significant role in scaffold efficiency and cardiac tissue engineering. As a result, multiple fabrication processes must be developed in order to generate the best scaffold for cardiac tissue creation [30,33].
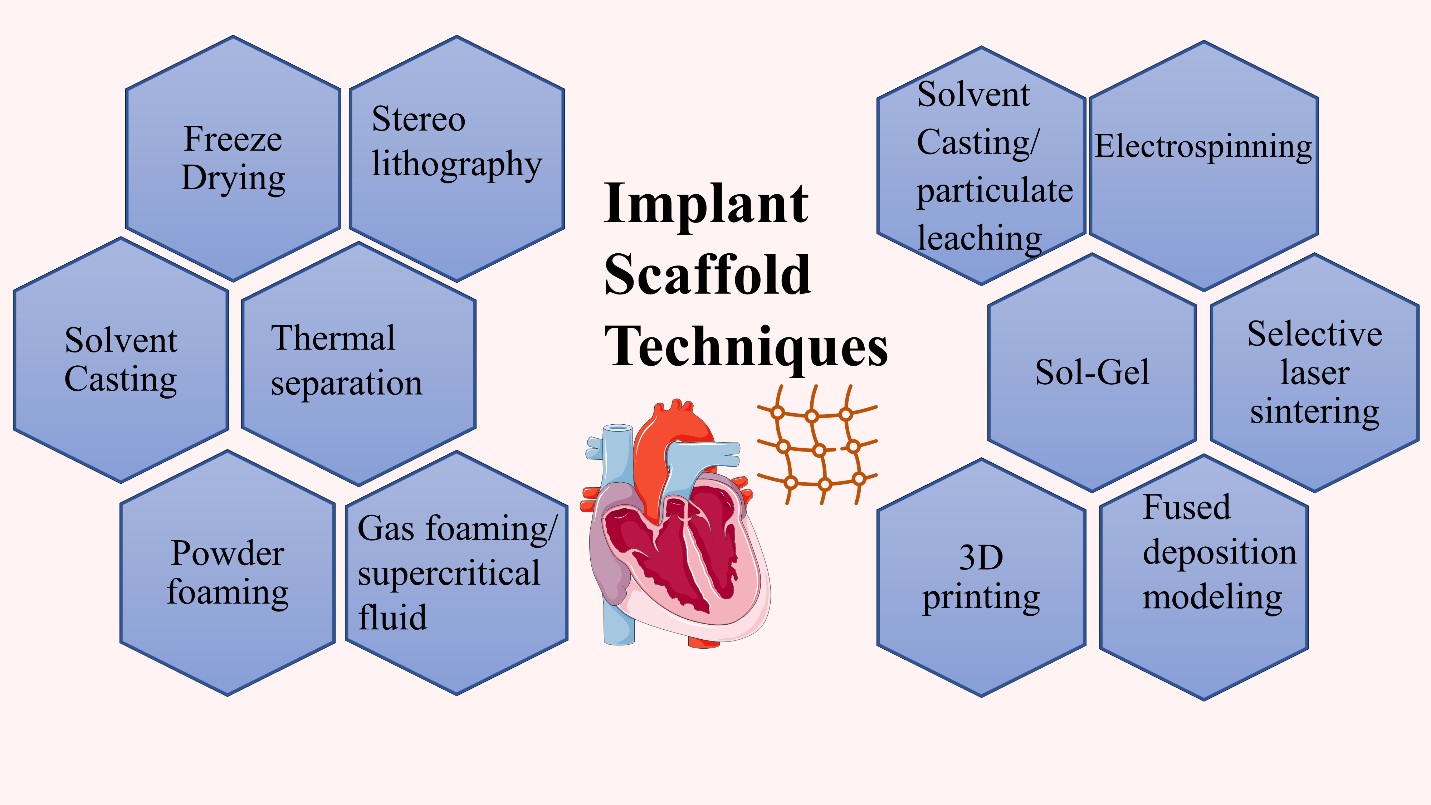
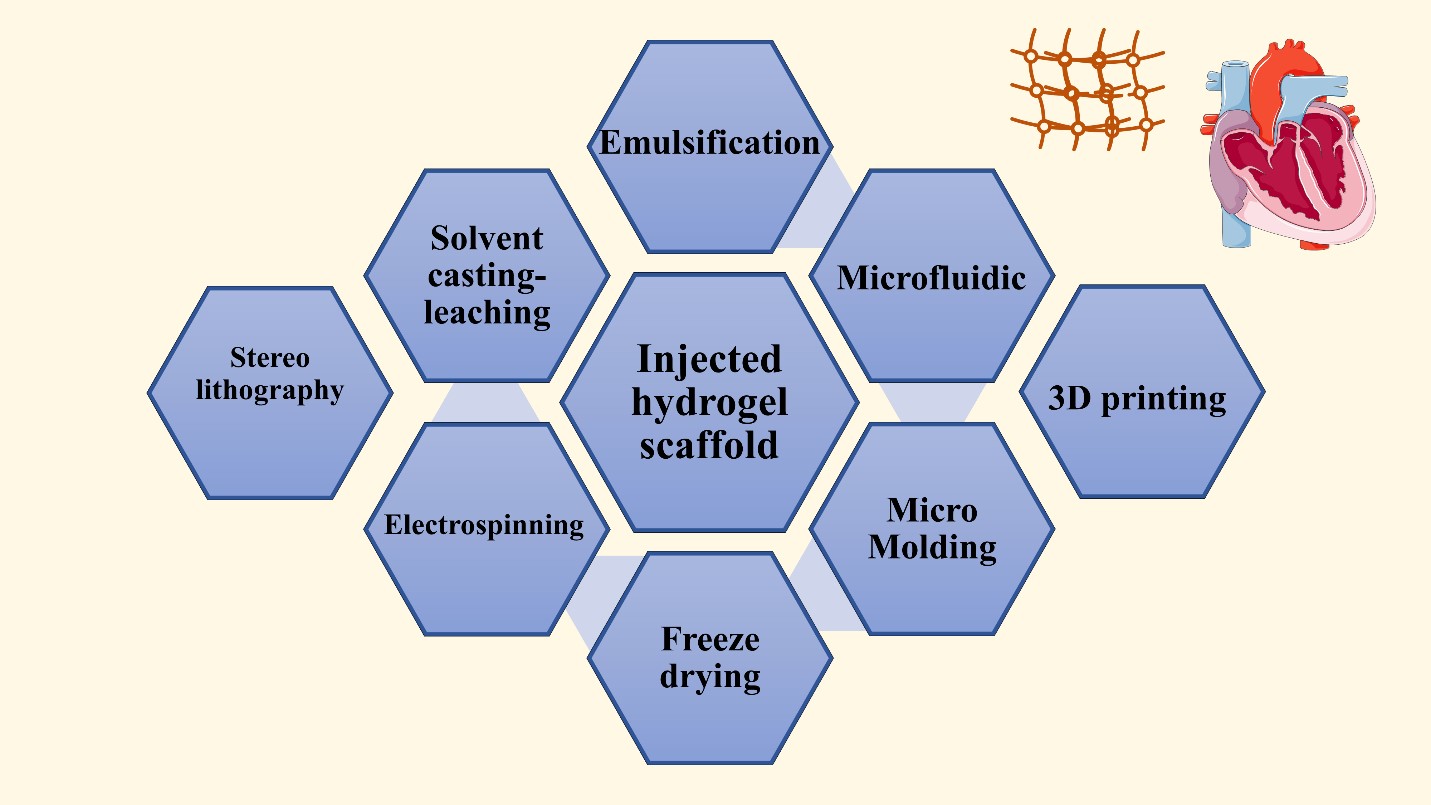
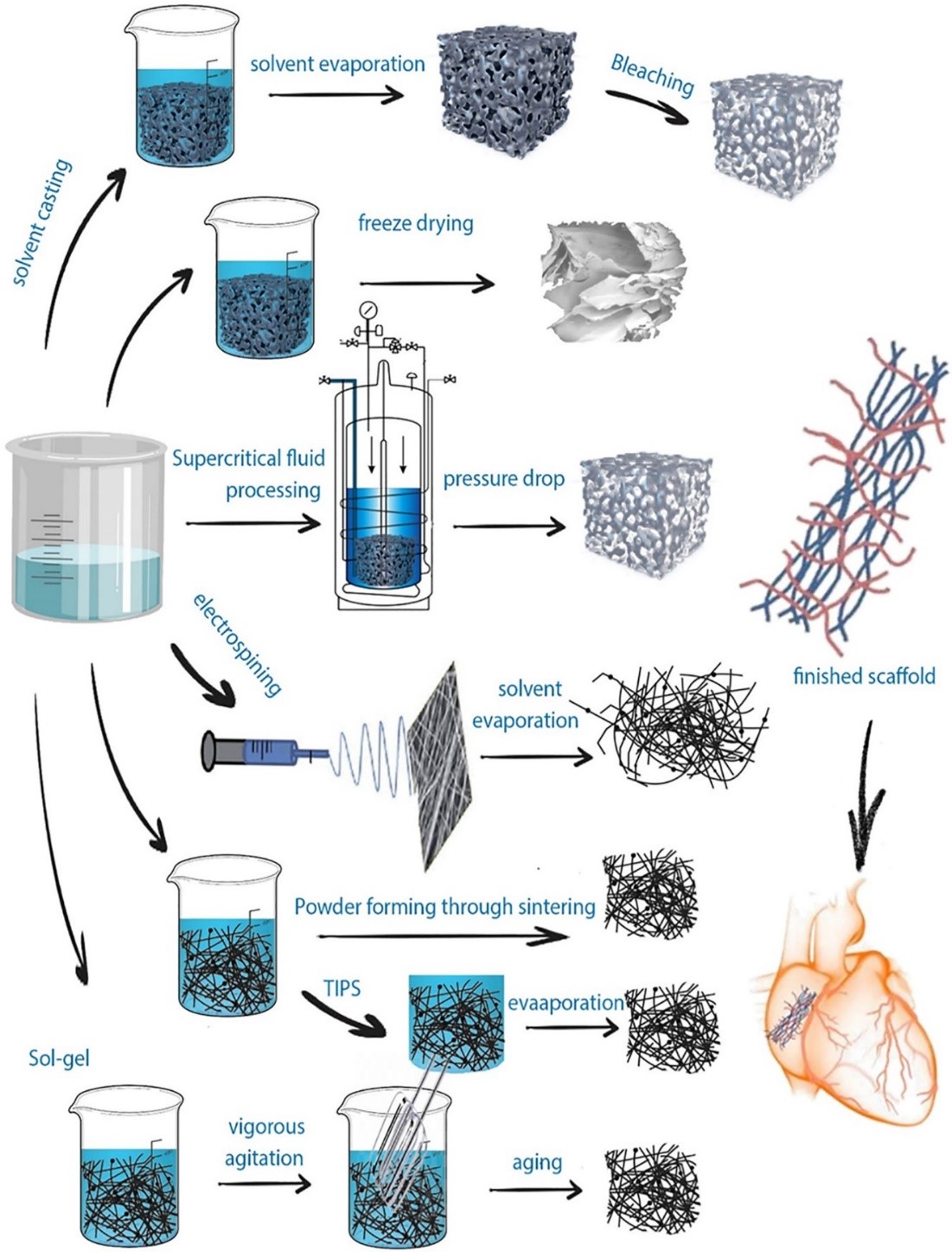
The fabrication techniques can be classified into implanted scaffolds and injected hydrogel scaffolds. In the implanted scaffolds they are classified into conventional and non-conventional fabrication techniques. The conventional techniques are illustrated in figures 12 and 13. and they include freeze-drying, electrospinning, solvent casting, solvent casting/particulate leaching, sol-gel method, thermal separation, and foaming techniques. While the non--conventional methods are selective laser sintering, 3D printing, and stereolithography. On the other hand, examples of the injected hydrogel scaffolds are emulsification, freeze-drying, emulsification-freeze drying, microfluidic, micro-molding,3D printing, and solvent casting leaching as shown in Figure 12 [70].
The solvent casting/particulate leaching produces a porous scaffold, and the manufacturing depends on pouring the mixture into a mold after dissolving the polymers in a solvent with high volatile properties in the presence of a suitable porogen. A temperature variation can be a major factor in scaffold fabrication in a method known as thermally induced phase separation. It permits polymer solution phase separation depending on polymer concentration. It separates polymers into high concentrated polymers and low concentrated polymers. On the other hand, freeze-drying can be used without a high temperature applied as in the thermally induced phase separation. The freeze-drying process can produce porous scaffolds via using a polymer solution that is frozen and then this frozen solution undergoes freeze-dried under a vacuum. Another commonly used fabrication technique is electrospinning, where an electric current is applied to a polymer solution added in a needle either a single needle or a double needle. The double needle is usually used to form a core/shell fiber. The produced fiber features and morphology can be controlled and altered via changes in humidity, temperature, needle shape, and size.In addition to the applied electrical current and collector shape. Every technique possesses its advantages and disadvantages,but there are common drawbacks in the conventional techniques including the inability to produce fully homogeneous pores, obstacles to controlling the scaffold geometry,and the use of organic solvents [30], [70]. Because of these limitations, unconventional techniques emerged such as 3D printing, and laser ablation which are computer-based techniques. They overcome the conventional methods and introduce scaffolds successful for cardiac tissue regeneration.Selective laser sintering, a commonly used technique,depends on applying laser beams as infrared or CO2 lasers, which leads to fusion and solidification of the applied polymers.This method allows the formation of a complex scaffold consisting of layers above each other [30,70].
Conclusive Remarks
Heart diseases are the major cause of death worldwide and the situation gets worse over time. The problem beyond this is that the cardiomyocytes do not have the ability to regenerate and compensate for the dead cells. Therefore,when there is an injury or a disease in the heart such as myocardial infarction, it leads to scar tissue formation but not regeneration, where the cardiomyocytes are nonfunctional and lack contractile activity.
Scaffold construction approaches combining biodegradable polymers, stem cells, progenitor or adult cardiac cells, and biomolecules have opened up a new landscape in the field of cardiac tissue regeneration. Scaffolds also allow for drug delivery with fewer cytotoxicity and side effects.In this review, we highlighted some extremely encouraging outcomes in terms of the development of scaffolds for cardiac tissue engineering. But as of this now, there isn't a perfect biodegradable polymer that meets all the criteria for creating cardiac tissue.
Diverse fabrication techniques and forms have been widely used in research. However, new techniques are required to pave the way for more sophisticated cardiac regeneration and to overcome emerging limitations. For instance,using the 3D bioprinting fabrication method and combining them with suitable types of stem cells, such as the iPSCs. Additionally, this can be combined with multi--biomaterials and new growth factors, such as those derived from plant extract, where every member will serve for specific purposes to achieve better regeneration.
Additionally, numerous trials have been used to promote vascularity within the cardiac patches, frequently by adding vasculogenic substances, but it is still difficult to produce big perfusable blood vessels. Also, further safety and toxicity tests are needed.
On the other hand, further investigations are still required for the promising established scaffolds in order to reach clinical trial phases. Furthermore, more studies are essential to be able to introduce a product that can reach the market to save the lives of patients suffering from myocardial infarction and heart failure.
- K Breckwoldt, F Weinberger, T Eschenhagen (2016)“Heart regeneration,” Biochim Biophys Acta Mol Cell Res 1863: 1749-59.
- H Hashimoto, EN Olson, R Bassel-Duby (2018) “Therapeutic approaches for cardiac regeneration and repair,” Nat Rev Cardiol 15: 585-600.
- M Mazzola, E Di Pasquale (2020) “Toward Cardiac Regeneration:Combination of Pluripotent Stem Cell-Based Therapies and Bioengineering Strategies,” Frontiers in Bioengineering and Biotechnology 8.
- A Singh, A Singh, D Sen (2016) “Mesenchymal stem cells in cardiac regeneration: A detailed progress report of the last 6 years (2010-2015),” Stem Cell Research and Therapy 7:1.
- LC Liew, BX Ho, BS Soh (2020) “Mending a broken heart: Current strategies and limitations of cell-based therapy,”Stem Cell Res Ther 11: 1-15.
- C Cristallini, E Vitale, C Giachino, R Rastaldo (2020) “Nanoengineering in cardiac regeneration: Looking back and going forward,” Nanomaterials 10: 1-28,
- SM Nasr et al. (2020) “Biodegradable nanopolymers in cardiac tissue engineering: From concept towards nanomedicine,” Int J Nanomedicine 15- 4205-24.
- R Verjans, M van Bilsen, B Schroen (2020) “Reviewing the limitations of adult mammalian cardiac regeneration:Noncoding RNAs as regulators of cardiomyogenesis,” Biomolecules 10: 1-23.
- O Bergmann et al. (2015) “Dynamics of Cell Generation and Turnover in the Human Heart,” Cell 161: 1566-75.
- SE Senyo et al. (2013) “Mammalian heart renewal by pre-existing cardiomyocytes,” Nature 493: 433-6.
- ER Porrello et al. (2011) “Transient regenerative potential of the neonatal mouse heart,” Science (1979) 331:1078-80.
- KD Poss, LG Wilson, MT Keating (2002) “Heart regeneration in zebrafish,” Science (1979) 298: 2188-90.
- C Jopling, E Sleep, M Raya, M Martí, A Raya, JCI Belmonte (2010) “Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation,” Nature 464:606-9.
- A Lepilina et al. (2006) “A Dynamic Epicardial Injury Response Supports Progenitor Cell Activity during Zebrafish Heart Regeneration,” Cell 127: 607-19.
- BJ Haubner et al. (2012) “Complete cardiac regeneration in a mouse model of myocardial infarction,” Aging 4:966-77.
- DM Bryant, CC O’Meara, NN Ho, J Gannon, L Cai,RT Lee (2015) “A systematic analysis of neonatal mouse heart regeneration after apical resection,” J Mol Cell Cardiol 79:315-8.
- K Kikuchi, KD Poss (2012) “Cardiac regenerative capacity and mechanisms,” Annual Review of Cell and Developmental Biology 28: 719-41.
- VFM Segers, RT Lee (2008) “Stem-cell therapy for cardiac disease,” Nature 451: 937-42.
- AH Nguyen et al. (2019) “Cardiac tissue engineering:State-of-the-art methods and outlook,” J Biol Eng 13: 57.
- S Gupta, A Sharma, S Archana, RS Verma (2021) “Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery,” Stem Cell Rev Rep 17:1666-94,
- R Augustine et al. (2021) “Stem cell-based approaches in cardiac tissue engineering: controlling the microenvironment for autologous cells,” Biomedicine and Pharmacotherapy 138: 111425.
- X Chu et al. (2022) “Strategies for constructing pluripotent stem cell- and progenitor cell-derived three-dimensional cardiac micro-tissues,” J Biomed Mater Res A 110:488-503.
- SA Doppler, MA Deutsch, R Lange, M Krane (2013) “Cardiac regeneration: Current therapies-future concepts,” JThorac Dis 5: 683-97.
- U Galderisi, G Peluso, G Di Bernardo (2022) “Clinical Trials Based on Mesenchymal Stromal Cells are Exponentially Increasing: Where are We in Recent Years?,” Stem Cell Reviews and Reports 18: 23-36.
- IR Murray et al. (2019) “International Expert Consensus on a Cell Therapy Communication Tool: DOSES,” Journal of Bone and Joint Surgery - American 101: 904-11.
- P Kc, Y Hong, G Zhang (2019) “Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair:Advantages and challenges,” Regen Biomater 6: 185-99.
- HC Ott et al. (2008) “Perfusion-decellularized matrix:Using nature’s platform to engineer a bioartificial heart,”Nat Med 14: 213-21.
- JM Wainwright et al. (2010) “Preparation of cardiac extracellular matrix from an intact porcine heart,” Tissue Eng Part C Methods 16: 525-32,
- PL Sánchez et al. (2015) “Acellular human heart matrix:A critical step toward whole heart grafts,” Biomaterials 61: 279-89.
- P Chandika et al. (2020) “Recent advances in biological macromolecule based tissue-engineered composite scaffolds for cardiac tissue regeneration applications,” Int J Biol Macromol 164: 2329-57.
- R Chaudhuri, M Ramachandran, P Moharil, M Harumalani,AK Jaiswal (2017) “Biomaterials and cells for cardiac tissue engineering: Current choices,” Materials Science and Engineering C 79: 950-7.
- Z Cui, B Yang, RK Li (2016) “Application of Biomaterials in Cardiac Repair and Regeneration,” Engineering 2:141-8.
- S Trombino, F Curcio, R Cassano, M Curcio, G Cirillo, F Iemma (2021) “Polymeric biomaterials for the treatment of cardiac post-infarction injuries,” Pharmaceutics 13: 7.
- P Menasché (2018) “Cell therapy trials for heart regeneration—lessons learned and future directions,” Nat Rev Cardiol 15: 659-71.
- J de S. Rebouças, NS Santos-Magalhães, FR Formiga (2016) “Cardiac regeneration using growth factors: Advances and challenges,” Arq Bras Cardiol 107: 271-5.
- BD Ulery, LS Nair, CT Laurencin (2011) “Biomedical applications of biodegradable polymers,” J Polym Sci B Polym Phys 49: 832-64.
- W qiang Wu, S Peng, Z yuan Song, S Lin (2019) “Collagen biomaterial for the treatment of myocardial infarction:an update on cardiac tissue engineering and myocardial regeneration,”Drug Deliv Transl Res 9: 920-34.
- YL Yang, S Motte, LJ Kaufman (2010) “Pore size variable type I collagen gels and their interaction with glioma cells,”Biomaterials 31: 5678-88.
- R Ravichandran et al. (2015) “Functionalised type-I collagen as a hydrogel building block for bio-orthogonal tissue engineering applications,” J Mater Chem B 4: 318-26.
- S Mirsadraee et al. (2006) “Development and characterization of an acellular human pericardial matrix for tissue engineering,” Tissue Eng 12: 763-73.
- MB Ariganello, RS Labow, JM Lee (2010) “In vitro response of monocyte-derived macrophages to a decellularized pericardial biomaterial,” J Biomed Mater Res A 93: 280-8,
- T Xu, P Molnar, C Gregory, M Das, T Boland, JJ Hickman (2009) “Electrophysiological characterization of embryonic hippocampal neurons cultured in a 3D collagen hydrogel,”Biomaterials 30: 4377-83.
- S Mirsadraee et al. (2007) “Biocompatibility of Acellular Human Pericardium,” Journal of Surgical Research 143:407-14.
- QA Majid et al. (2020) “Natural Biomaterials for Cardiac Tissue Engineering: A Highly Biocompatible Solution,”Front Cardiovasc Med 7.
- YL Lin, CP Chen, CM Lo, HS Wang (2016) “Stiffness-controlled three-dimensional collagen scaffolds for differentiation of human Wharton’s jelly mesenchymal stem cells into cardiac progenitor cells,” J Biomed Mater Res A 104: 2234-42.
- H Sun et al. (2017) “Carbon nanotube-incorporated collagen hydrogels improve cell alignment and the performance of cardiac constructs,” Int J Nanomedicine 12:3109-20.
- H Yu, H Zhao, C Huang, Y Du (2017) “Mechanically and Electrically Enhanced CNT-Collagen Hydrogels as Potential Scaffolds for Engineered Cardiac Constructs,” ACS Biomater Sci Eng 3: 3017-21.
- P Joanne et al. (2016) “Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy,” Biomaterials 80: 157-68.
- A Elamparithi, AM Punnoose, SFD Paul, S Kuruvilla (2017) “Gelatin electrospun nanofibrous matrices for cardiac tissue engineering applications,” International Journal of Polymeric Materials and Polymeric Biomaterials 66: 20-7.
- G Yang et al. (2016) “Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells,” PeerJ 1-22.
- M Alonzo, S AnilKumar, B Roman, N Tasnim, B Joddar (2019) “3D Bioprinting of cardiac tissue and cardiac stem cell therapy,” Translational Research 211: 64-83.
- D Kai, SS Liow, XJ Loh (2015) “Biodegradable polymers for electrospinning: Towards biomedical applications,”Materials Science and Engineering C 45: 659-70
- JT Kim et al. (2015) “A fibrin-supported myocardial organ culture for isolation of cardiac stem cells via the recapitulation of cardiac homeostasis,” Biomaterials 48: 66-83.
- L Melly et al. (2019) “Stimulation of Cardiac Angiogenesis through Growth Factors with a Fibrin Gel Delivery Platform,”The Journal of Heart and Lung Transplantation 38:S246.
- BV Slaughter, SS Khurshid, OZ Fisher, A Khademhosseini, NA Peppas (2009) “Hydrogels in regenerative medicine,” Advanced Materials 21: 3307-29
- T Hao et al. (2017) “Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair,”ACS Nano 11: 5474-88.
- B Xu, Y Li, B Deng, X Liu, L Wang, QL Zhu (2017) “Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats,” Exp Ther Med 13: 588-94.
- L Zhang et al. (2017) “Construction of vascularized pacemaker tissues by seeding cardiac progenitor cells and endothelial progenitor cells into Matrigel,” Life Sci 179: 139-46.
- C Bearzi et al. (2014) “PlGFMMP9-engineered iPS cells supported on a PEGfibrinogen hydrogel scaffold possess an enhanced capacity to repair damaged myocardium,” Cell Death Dis 5: e1053.
- MC Ciuffreda et al. (2018) “Synthetic extracellular matrix mimic hydrogel improves efficacy of mesenchymal stromal cell therapy for ischemic cardiomyopathy,” Acta Biomater 70: 71-83.
- M Boffito, S Sartori, C Mattu, G Ciardelli (2016) Polyurethanes for Cardiac Applications. Elsevier Ltd.
- A Silvestri et al. (2014) “Biomimetic myocardial patches fabricated with poly(ε-caprolactone) and polyethylene glycol-based polyurethanes,” J Biomed Mater Res B Appl Biomater 102: 1002-13.
- V Chiono et al. (2014) “Polyurethane-based scaffolds for myocardial tissue engineering,” Interface Focus 4: 1-11.
- A D’Amore et al. (2016) “Bi-layered polyurethane –Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model,” Biomaterials 107: 1-14.
- M Boffito et al. (2018) “Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells,” PLoS One 13:1-21.
- N Baheiraei et al. (2016) “Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiacpatch, part I: Synthesis, characterization, and myoblast proliferation and differentiation,” J Biomed Mater Res A 104:775-87.
- D Bezuidenhout, DF Williams, P Zilla (2015) “Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices,” Biomaterials 36: 6-25.
- SJ Stachelek et al. (2006) “Cholesterol-modified polyurethane valve cusps demonstrate blood outgrowth endothelial cell adhesion post-seeding in vitro and in vivo,” Annals of Thoracic Surgery 81: 47-55.
- M Zamani, MP Prabhakaran, ES Thian, S Ramakrishna (2015) “Controlled delivery of stromal derived factor-1α from poly lactic-co-glycolic acid core-shell particles to recruit mesenchymal stem cells for cardiac regeneration,” J Colloid Interface Sci 451: 144-52.
- M Roshandel, F Dorkoosh (2021) “Cardiac tissue engineering,biomaterial scaffolds, and their fabrication techniques,”Polym Adv Technol 32: 2290-305.

Figures at a glance