Modified Stomach-Partitioning Gastrojejunostomy with Jejunal Pouch for Gastric Outlet Obstruction
Received Date: April 14, 2025 Accepted Date: May 14, 2025 Published Date: May 17, 2025
doi:10.17303/jspcr.2025.7.102
Citation: Keisuke Ida, PhD; Shinjiro Kobayashi; Satoshi Koizumi, PhD; Shinya Mikami, PhD; Takehito Otsubo, PhD (2025) Modified Stomach-Partitioning Gastrojejunostomy with Jejunal Pouch for Gastric Outlet Obstruction. J Surg Proce Case Rep 7: 1-8
Abstract
Early stable oral intake is essential in malignancy-related gastric jejunostomy for gastric outlet obstruction (GOO). A modified stomach-partitioning gastrojejunostomy with jejunal pouch (MSGJstomy) technique was developed to anastomose the jejunal pouch with an incompletely dissected stomach. Patients who had undergone malignancy-related gastrojejunostomy for GOO between 2013 and 2023 were included. We compared surgical outcomes between an MSGJstomy and a simple anastomosis group in which the stomach and jejunum were simply anastomosed laterally via the anterior colonic route. The mean postoperative days (PODs) after starting a liquid diet were 4.4 ± 1.2 and 8.6 ± 1.3 days, respectively, being significantly shorter in the MSGJstomy group (P = 0.0288). Patients consumed at least 50% of a soft diet for 8.1 ± 1.4 and 12.9 ± 1.6 PODs, respectively (P = 0.0339). The simple anastomosis group experienced five (35.7%) cases of delayed gastric emptying (P = 0.0058).
Keywords: Gastric Bypass; Gastric Obstruction; Jejunal Pouch; Stomach-Partitioning
Introduction
Gastric outlet obstruction (GOO), which occurs as a complication of peptic ulcers and acute pancreatitis and in advanced gastric and pancreatic cancers, significantly reduces patient quality of life [1]. In GOO, owing to malignant disease, patient prognosis is severely compromised because chemotherapy is difficult to administer. If radical surgery is not possible, a gastric jejunal bypass is performed. Gastric jejunal bypass is the most common technique in which the stomach is incompletely dissected, and the jejunum is anastomosed [2]. However, in some cases, food intake cannot be easily maintained postoperatively. This may be due to anastomosis formation between the thickened gastric wall, as a result of edema, and the atrophic small intestine through which food does not pass owing to prolonged GOO. Therefore, we developed a modified stomach-partitioning gastrojejunostomy with a jejunal pouch (MSGJstomy) technique to secure the diameter of the anastomosis. The results were compared with those obtained using conventional methods.
Materials and Methods
Patients who had undergone gastric jejunal bypass for GOO owing to malignant disease at our hospital between January 1, 2013, and December 31, 2023, 37 patients were included in the study. The patients were divided into two groups, namely, an MSGJstomy group, in which MSGJstomy was performed, and a simple anastomosis group, in which the stomach and jejunum were simply anastomosed side by side via the anterior colonic route. Surgical outcomes were compared between the two groups. Patient characteristics were compared in terms of age (years), sex (male %), body mass index (BMI) (kg/cm2), American Society of Anesthesiologists physical status score (ASA-PS), preoperative prognostic nutritional index, and the preoperative prealbumin level (mg/dL). Surgical outcomes were compared in terms of operative time (min), blood loss (mL), postoperative day (POD) when a liquid diet was started, POD when at least 50% of a soft diet could be consumed, a postoperative complication rate of Clavien-Dindo classification II or higher, the presence of delayed gastric emptying (DGE), and the number of hospitalized PODs.
DGE
DGE was defined according to the International Study Group of Pancreatic Surgery definition of impaired evacuation of gastric contents after pancreatoduodenectomy [3]. In this study, DGE was defined as (i) continuous insertion or reinsertion of a nasogastric tube beyond three PODs or (ii) an inability to start oral intake after POD 7.
MSGJstomy Procedure
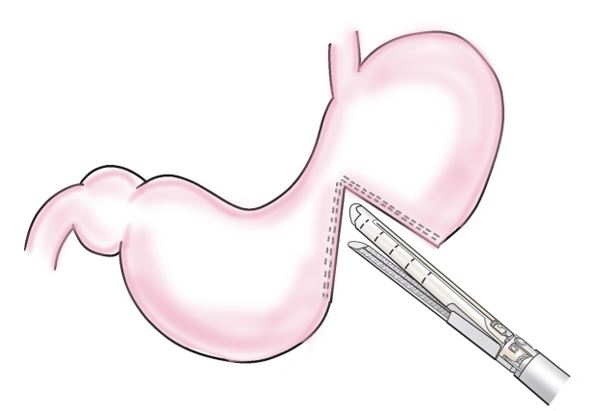
Step 1: The contralateral greater curvature of the gastric horn was the site of the planned anastomosis, and 2–3 marginal blood vessels in the same area were ligatured and dissected. The stomach was then incompletely dissected with a stapling device (3.5 mm/80 mm), leaving a 2–3 cm gastric angle on the lesser fold side. A serosal muscle layer suture was added to the suture line on the anorectal side using a 4-0 absorbable thread to cover the staple (Figure 1).
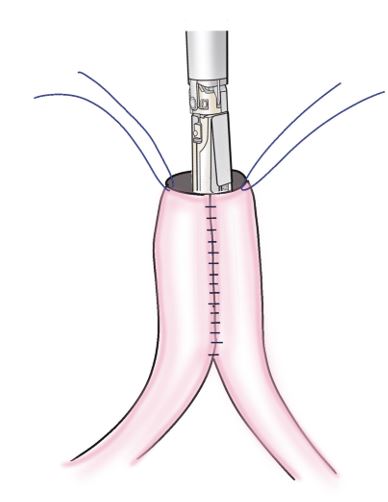
Step 2: The jejunal wall 30–40 cm from the ligament of Treitz was inserted into an entry hole through a longitudinal incision on the contralateral side of the mesentery. A stapling device (3.5 mm/80 mm) was inserted through an entry hole into the oral and anal lumens. The jejunum was bent and suture-separated, such that the suture line was on the contralateral side of the mesentery, to create a jejunal pouch (Figure 2).
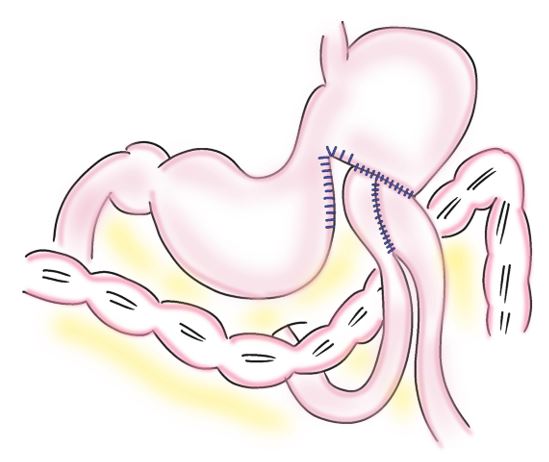
Step 3: The jejunal pouch was anastomosed to the stomach via the anterior route of the colon. An Albert-Lembert anastomosis was performed between an incompletely dissected oral segment of the stomach and the jejunal pouch using a 4-0 absorbable thread. The diameter of the anastomosis should match that of the jejunal pouch. The staple line of the gastric transection, which was not anastomosed, was sutured with a 4-0 absorbable thread in the serosal muscularis layer (Figure 3).
Simple Anastomosis Procedure
The jejunum 30–40 cm from the ligament of Treitz was elevated via the anterior colonic route. An Albert-Lembert anastomosis was performed between the contralateral side of the mesenteric attachment of the jejunum and the greater curvature of the stomach in the longitudinal and reversed peristaltic directions. A Brown anastomosis was then performed to laterally anastomose the jejunum.
Statistical Analysis
All Statistical computations were performed using JMP® 14 (SAS Institute Inc., Cary, NC, USA). Descriptive data are reported as mean (standard deviation), median and range, or number of patients and percentage. Categorical variables were compared via Chi-squared test, and continuous variables via Student's t test and non-parametric Mann–Whitney U test. The significance level was set at 0.05.
Results
During the study period, 38 gastric jejunal bypass surgeries were performed at our hospital for GOO caused by a malignant disease. Of these, we excluded 10 cases in which the procedure was not routine and compared the surgical outcomes of 13 cases in the MSGJstomy group with 14 cases in the simple anastomosis group. Patient backgrounds showed no significant differences in age, sex, BMI, and ASA-PS.
The mean operative time and blood loss were 180.5 ± 18.8 min and 97.8 ± 47.8 mL in the MSGJstomy group and 205.1 ± 18.1 min and 135.9 ± 46.1 mL in the simple anastomosis group, respectively (P = 0.3560 and P = 0.5706, respectively). The mean postoperative days after starting the liquid diet were 4.4 ± 1.2 days in the MSGJstomy group and 8.6 ± 1.3 days in the simple anastomosis group, which was significantly shorter in the MSGJstomy group (P = 0.0288). The number of postoperative days when at least 50% of the soft diet could be consumed was also shorter in the MSGJstomy group (8.1 ± 1.4 days) than in the simple anastomosis group (12.9 ± 1.6 days, P = 0.0339). Postoperative complications of Clavien-Dindo classification II or higher occurred in 7 (50.0%) patients in the simple anastomosis group, whereas none occurred in the MSGJstomy group (P = 0.0007). The MSGJstomy group reported no cases of DGE, while the simple anastomosis group experienced 5 (35.7%) cases (P = 0.0058). The mean postoperative hospital stay was 13.7 ± 6.2 days in the MSGJstomy group and 29 ± 6.2 days in the simple anastomosis group (P = 0.0927, Table 1).
Discussion
GOO treatments include intestinal decompression with gastrostomy, endoscopic stenting, and gastric jejunal bypass [4]. Gastric jejunal bypass is further divided into surgical bypass (open and laparoscopic bypass), natural orifice translumenal endoscopic surgery (NOTES), and endoscopic bypass such as endoscopic ultrasound (EUS) bypass [5,6]. Wolfler first reported surgical bypass in 1881 when the Billroth II technique was applied to a gastric jejunal bypass for unresectable gastric cancer [7]. This technique involves a simple lateral anastomosis of the jejunum elevated via the anterior colonic route to the stomach and is still relatively common because of its simplicity. However, this technique is fraught with challenges, such as regurgitation of duodenal fluid into the stomach and stenosis associated with early invasion of the cancer into the anastomosis. In the early postoperative period, the anastomotic area becomes thickened owing to edema, which can lead to stagnation of gastric contents when the anastomotic diameter is small [8–11]. Improvement of gastrointestinal edema takes 7–14 days or longer, and delayed elimination of gastric contents can significantly reduce the quality of life by rendering oral intake impossible in a long postoperative period [12]. Devine’s technique to anastomose the jejunum to the antral stomach after the stomach is dissected has been reported to prevent postoperative DGE [13]. This technique can prevent early stenosis of the anastomosis resulting from cancer invasion; however, endoscopic observation of the dissected antral stomach is not possible, and accumulation of gastric juice occurs along with the risk of hemorrhage. To address these drawbacks, incomplete dissection of the greater curvature of the stomach while leaving the lesser curvature intact and anastomosis of the jejunum to the healthy portion have been reported [14].
The mortality rate within 30 days after palliative surgery for advanced cancer has been reported to be 11–12%, especially in pancreatic cancer, where approximately 30% of patients were reported to have died without oral intake after gastrojejunal bypass [15,16]. Among the cases reviewed in this study, two of the 14 patients who underwent simple gastrojejunal bypass died of the original disease without oral intake. The proximal jejunum, through which food cannot pass, tends to atrophy. Therefore, when the atrophic jejunum is anastomosed with the dilated and edematous stomach owing to impaired transit, the anastomotic diameter is at most that of the short axis of the jejunum, which may cause anastomotic stenosis until the edema is managed. Several studies have reported the advantages of jejunal pouch anastomosis over the Billroth II method in gastric and jejunal reconstruction following distal gastrectomy, including a lower incidence of gastric content stagnation. Moreover, it has been noted that reconstruction using a jejunal pouch allows for the creation of a wider anastomotic site, which may contribute to improved postoperative gastrointestinal function [17-19].
In MSGJstomy, forming a jejunum pouch creates room in the longitudinal direction of the jejunum, enabling a larger anastomosis than a lateral anastomosis. The sufficiently large anastomotic size prevents DGE even when edema develops at the anastomotic site, preventing stenosis and enabling stable early oral intake.
The main features of this method are as follows: (i) incomplete separation of the stomach limits the flow of food into the stenosis while leaving the lesser curvature side of the stomach to prevent gastric blow-out owing to accumulation of gastric juice and blood in the separated distal stomach, (ii) the addition of a jejunal pouch prevents gastric reflux of small intestinal fluid, and (iii) the gastric dissection and jejunal pouch can be easily performed with a stapler, and the gastric jejunal anastomosis can be sutured by hand to secure a large anastomotic opening. In cases of stenosis caused by pancreatic cancer on the anorectal side of the duodenal papilla, an endoscopic approach to the biliary stricture may be possible by keeping the stomach in incomplete dissection. The jejunal pouch has been reported to have a lower incidence of gastritis and reflux esophagitis resulting from reflux of the small intestinal fluid than the Billroth II method and is considered to reduce postoperative pericardial discomfort and enable stable food intake [20]. In addition, we consider that the use of a hand-sutured gastric jejunal anastomosis has the advantage of ensuring a large anastomotic size to prevent anastomotic edema and organic DGE owing to narrowing. In a comparison of laparoscopic gastrojejunostomy bypass outcomes, the operative time was shorter in our open surgery [21,22], while the start date of the postoperative solid food intake was the same. Therefore, in patients with GOO and a high surgical risk of carcinomatous conditions, our hospital performs open surgery in a shorter time.
This study has several limitations. First, it was a retrospective analysis conducted at a single institution, resulting in a limited sample size of patients with GOO owing to malignant disease. Only 27 patients were included, which may have reduced the statistical power and generalizability of the findings. Second, for the reasons mentioned above, bypass surgery at our institution is generally performed via open laparotomy rather than minimally invasive techniques. As a result, a comparative evaluation between open and laparoscopic approaches was not feasible in this study. Further prospective multicenter studies with larger cohorts are warranted to provide more robust evidence and to better elucidate the clinical utility and safety of MSGJstomy.
Conclusion
We report the surgical results of gastric jejunostomy bypass with MSGJstomy performed at our institution. Compared with conventional simple gastric jejunostomy, the time to start eating was shorter, and the incidence of postoperative intragastric stagnation was lower. This procedure may enable stable early oral intake, which is the most important issue in gastric jejunostomy bypass surgery, especially for malignant tumors.
Acknowledgment
None.
Conflicts of Interests
All authors declare no conflicts of interest
Funding
None.
Ethics Statement
The Institutional Review Board of St. Marianna University School of Medicine approved this study prior to the initiation of data collection and analysis (IRB no. 6722)
- Mohammed AA, Benmousa A, Almeghaiseeb I, Alkarawi M (2007) Gastric outlet obstruction. Hepatogastroenterology. 54: 2415–20.
- Kaminishi M, Yamaguchi H, Shimizu N, Nomura S, Yoshikawa A, Hashimoto M, et al. (1997) Stomach-partitioning gastrojejunostomy for unresectable gastric carcinoma. Arch Surg. 132: 184–7.
- Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. (2007) Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery.
- Chan SM, Dhir V, Chan YYY, Cheung CHN, Chow JCS, Wong IWM, et al. (2023) Endoscopic ultrasound-guided balloon-occluded gastrojejunostomy bypass, duodenal stent or laparoscopic gastrojejunostomy for unresectable malignant gastric outlet obstruction. Dig Endosc.
- Vanbiervliet G, Bonin EA, Garcès R, Gonzalez JM, Garnier E, Paul MC, et al. (2014) Gastrojejunal anastomosis using a tissue-apposing stent: a safety and feasibility study in live pigs. Endoscopy.
- Itoi T, Tsuchiya T, Matsunami Y, Tonozuka R, Ishii K, Tanaka R, et al. (2020) EUS-guided gastrojejunostomy: Double-balloon occlusion theory with experimental study (with video). J Hepatobiliary Pancreat Sci.
- Pappas TN (2022) The first 40 years of gastrojejunostomy: from Billroth to Murphy to Mayo. Ann Surg Open.
- Sampaio-Neto J, Branco-Filho AJ, Nassif LS, Broska AC, Kamei DJ, Nassif AT (2016) Complications related to gastric bypass performed with difference gastrojejunal diameters. Arq Bras Cir Dig.
- Tyndall SH, Memon MA, Lund RJ, Beck D, Fessenden J, Stegman MR, et al. (1999) The effect of the anastomotic size on gastric emptying after hemigastrectomy with Billroth II reconstruction. Eur J Gastroenterol Hepatol.
- Lewis CE, Jensen C, Tejirian T, Dutson E, Mehran A (2009) Early jejunojejunostomy obstruction after laparoscopic gastric bypass: case series and treatment algorithm. Surg Obes Relat Dis.
- Levine MS, Fisher AR, Rubesin SE, Laufer I, Herlinger H, Rosato EF (1991) Complications after total gastrectomy and esophagojejunostomy: radiologic evaluation. AJR Am J Roentgenol.
- Tonoda S (1977) The experimental studies on the effect of suture materials for the wound healing of one-layer colonic anastomosis. The Japanese Journal of Gastroenterological Surgery (Nihon Shokaki Geka Gakkai Zasshi).
- Devine HB (1925) Basic principle and supreme difficulties in gastric surgery. Surg Gynecol Obstet. 40: 1–16.
- Kajitani T, Hisano K, Nishi M (1971) Gendai gekagaku taikei 35B. Nakayama Shoten, Tokyo.
- Miner TJ, Brennan MF, Jaques DP (2004) A prospective, symptom related, outcomes analysis of 1022 palliative procedures for advanced cancer. Ann Surg. 240: 719–27.
- Matsuda M, Watanabe G, Hashimoto M, Sasaki K (2013) Indications for gastroenteric bypass surgery in patients with unresectable pancreatic cancer with duodenal obstruction. Journal of the Japan Pancreas Society. 28: 62–6.
- Chen G, Cheng L, Liu L, Luo G, Li M, Wen Y, et al. (2022) Comparison of gastric-jejunum pouch anastomosis and Billroth-II reconstructions after distal gastrectomy: a propensity score matching analysis. Ann Surg Treat Res.
- Nomura E, Shinohara H, Mabuchi H, Sang-Woong L, Sonoda T, Tanigawa N (2004) Postoperative evaluation of the jejunal pouch reconstruction following proximal and distal gastrectomy for cancer. Hepatogastroenterology. 51: 1561-6.
- Chen G, Wu J, Zhang H, Wen Y, Luo G, Chen Z, et al. (2022) Addition of Jejunal Lateral Anastomosis is Not Necessary for Gastric-Jejunum Pouch Anastomosis following Distal Gastrectomy: A Propensity-Score Matching Analysis. J Invest Surg. 35: 1263-68.
- Gong J, Wang B, Wang J, Li Y, Cao Y, Li W, et al. (2022) Continuous jejunal pouch and residual stomach anastomosis combined with jejunal lateral anastomosis: an improved method of gastrointestinal reconstruction following distal gastrectomy. J Invest Surg.
- Mittal A, Windsor J, Woodfield J, Casey P, Lane M (2004) Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg.
- Navarra G, Musolino C, Venneri A, De Marco ML, Bartolotta M (2006) Palliative antecolic isoperistaltic gastrojejunostomy: a randomized controlled trial comparing open and laparoscopic approaches. Surg Endosc.

Tables at a glance
Figures at a glance