Lateral, Anterior, and Posterior Displacement of the Uterus and its Effect on Dysmenorrhea
Received Date: April 25, 2023 Accepted Date: May 25, 2023 Published Date: May 29, 2023
doi: 10.17303/jwhg.2023.10.102
Citation: Ozgul Muneyyirci-Delale, Frida Popilevsky, Nawras Zayat, Ranjitha Vasa (2023) Lateral, Anterior, and Posterior Displacement of the Uterus and its Effect on Dysmenorrhea. Womens Health Gyn 10: 1-13
Abstract
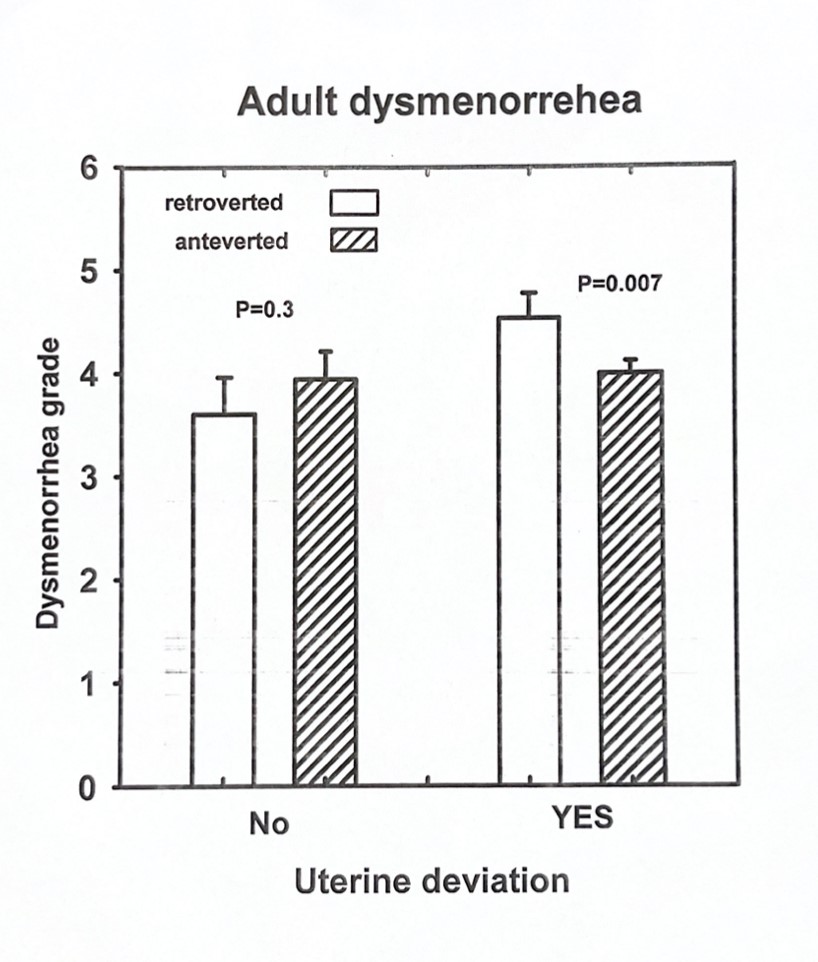
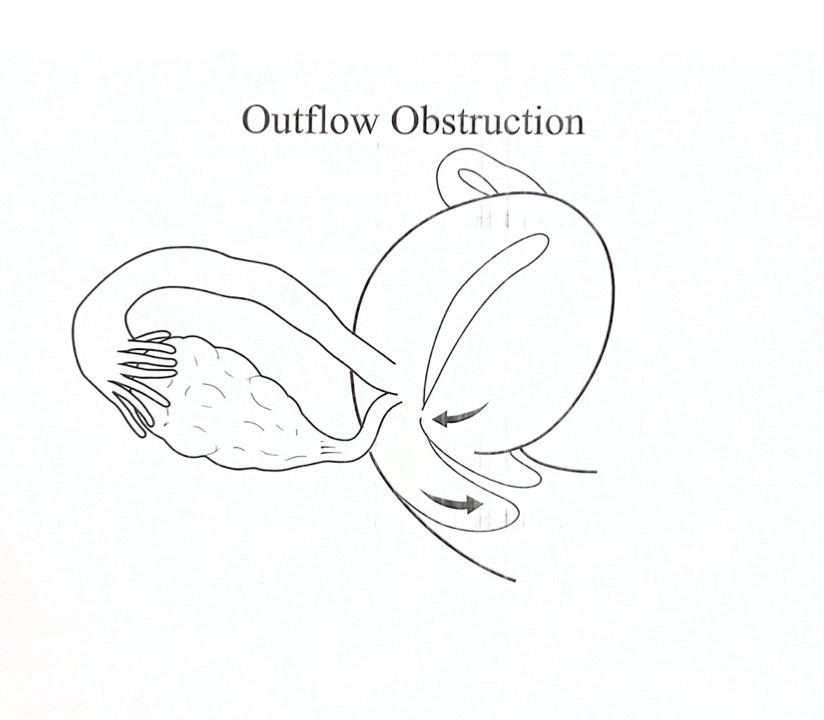
To examine whether lateral and anterior/posterior displacement of the uterus and cervix is associated with teenage dysmenorrhea and a maximum lifetime severity of dysmenorrhea. In this retrospective clinical study conducted at an academic university hospital, 1500 patient charts were reviewed retrospectively after IRB approval. Three hundred twenty-three patient records met the criteria of the presence of teenage dysmenorrhea and lateral displacement of the cervix and uterus. The primary outcome measures were the maximum severity of dysmenorrhea in a lifetime and the teenage severity of dysmenorrhea. Using factorial ANCOVA, the Teenage Severity of Dysmenorrhea (tsev) is significantly associated with a retroverted uterus than an anteverted uterus (4.24 ± 0.24 vs. 3.35 ± 0.12, p=0.001) in those patients with lateral displacement of the uterus. When the maximum severity of dysmenorrhea in the patient’s lifetime (maxsev) was considered with lateral displacement of the uterus, the severity was greater in patients with retroverted compared to anteverted uteri (4.53±0.19 vs.3.94±0.1, p = 0.007). In the absence of lateral uterine displacement, anterior/posterior displacement did not influence the maximum severity of dysmenorrhea (3.99 ± 0.21 vs. 3.60 ± 0.29, p+0.3). In conclusion, the severity of pain associated with a retroverted or anteverted uterus depends on whether the uterus is also displaced laterally. With lateral displacement of the uterus or cervix or the combination of the two, an outflow obstruction might occur, resulting in increased uterine contraction and possible retrograde menstruation.
Keywords: Dysmenorrhea, Endometriosis, Cervical Displacement
Capsule The severity of pain associated with a retroverted or anteverted uterus depends on whether the uterus is also displaced laterally.
Introduction
Dysmenorrhea is defined as painful menstruation.Dysmenorrhea can be sorted into two categories: primary and secondary; the former title is used when there is no visible pelvic pathology to account for dysmenorrhea [1]. Primary dysmenorrhea occurs more frequently during adolescence and early twenties [1-4], the prevalence declines after age 30 and more after age 35. Among adolescent females, the prevalence of dysmenorrhea ranges from 20-93 percent [4-7]. However, only 15-29 percent of females seek medical advice for menstrual pain [2,3]. 10-15 percent of women with primary dysmenorrhea suffer severely enough to render them incapacitated for 1-3 days each month. Many adolescents report limitations on daily activities due to menstrual pain, such as missing school, inability to participate in sporting events, and avoiding social activities [2,6]. In fact, primary dysmenorrhea takes a mental toll onthose who are impacted. Sahin et al. determined that among adolescents between the ages of 12-18, dysmenorrhea was associated with increased depression scores and decreased overall quality of life; however, anxiety levels were not impacted [9]. Most recently, MacGregor et al. provided evidence that adolescents with dysmenorrhea had statistically significantly increased depression, anxiety, and poor quality-of-life scores[51]. Therefore, early intervention and management of primary dysmenorrhea is key because the chronic abdominal pain that patients face disrupts other facets of their daily lives such as their mental, physical, and even social health.Secondary dysmenorrhea is defined as painful menstrual cycles due to a pathology of the pelvic origin or a defined medical condition [8]. Endometriosis is the top cause of secondary dysmenorrhea and should be suspected in patients who continue to have persistent dysmenorrhea despite management with NSAIDs and hormonal treatments [8]. Although there are multiple standard therapies for dysmenorrhea,they have not been well studied [1].
Dysmenorrhea is attributed to increase in prostaglandin production in the endometrium; increase in prostaglandins causes vasoconstriction of the blood vessels in the uterus which elicits lower abdominal pain [8]. NSAIDs are first line therapy as they inhibit prostaglandin synthetase, and they are indicated about 1 to 2 days before the resumption of menses and continued for 2 days thereafter. Side effects of NSAIDs include stomach aches, stomach ulcers, headaches, drowsiness. For patients who fail to respond to NSAIDs, oral contraceptive pills or medroxyprogesterone are added to mitigate pain. Side effects of oral contraceptive pills and medroxyprogesterone include irregular vaginal bleeding, weight gain, headache [8].
Alternative treatments do exist to manage dysmenorrhea such as application of topical heat at 38.9°C for 12 hours per day. In fact, Akin et al. provided evidence that combination of heated patch plus Ibuprofen had significantly greater pain relief compared to either intervention alone [10]. Transcutaneous electrical nerve stimulation (TENS) which has been shown to provide moderate relief in 40 to 60% of patients [11]. Acupuncture has led to improvement in lower abdominal pain in 91% of patients, and use of daily Thiamine 100mg for 90 days completely resolved lower abdominal pain symptoms in up to 2 months for 87% of patients [12]. Physical activity in the form of exercise has been shown to mitigate dysmenorrhea through increase in progesterone and reduction in pain mediators; however, the frequency, type, and length of exercise has yet to be accurately distilled [13]. Interestingly, diets rich in processed meats, oils, and other inflammation-promoting foods can compound dysmenorrhea [13].
With most women impacted by primary dysmenorrhea, the intensity of menstrual cramps and associated symptoms are proportional to the amount of prostaglandin F2x (PGF2x) released [14]. However, some patients with normal laparoscopic findings and severe dysmenorrhea do not have elevated PGF2x to account for the severe cramping. [14, 15]. Pulkkinen suggested that diminished uterine blood flow and anoxic pain are the underlying pathophysiologic mechanisms of primary dysmenorrhea [16]. Further, the expulsion of the endometrial contents through a displaced or even stenosed cervix can cause the uterus to contract with more strength and thus cause an increase in pain with menses [17]. In fact, Stratton and Berkley propose that endometriotic lesions can develop their own nerve supply which leads to various CNS stimulation contributing to differing pain sensations [19]. Specifically, Bulletti et al. found that the frequency, amplitude, and basal pressure tone of uterine contractions in women with endometriosis were higher than in those without [20]. Thus, the severe dysmenorrhea of endometriosis patients may result from abnormal uterine contractions and higher concentrations of prostaglandins in menstrual blood, as mentioned previously [20, 21]. Lastly, endometrial-tissue that grows outside the uterus, as seen with endometriosis, can thicken, breakdown as is expected during the menstrual cycle; however, this tissue especially becomes trapped when growing outside the uterus [19]. In fact, this tissue can become irritated, lead to scar tissue and adhesions which can lead to bands of tissue that can adhere to each other, further compounding pain [19].
More specifically, the uterocervical angle must be examined when discussing the impact of cervical displacement on dysmenorrhea. The uterocervical angle (UCA) is between the cervical canal and the uterus frontal wall. Dziadosz et al. recently reported that with a large UCA, uterine contents are more easily displaced to the cervix; likewise, in a study by Sahin et al., it was determined that the narrower anterior uterocervical angle is strongly associated with primary dysmenorrhea [22,23]. A narrower UCA will increase this resistance; thus, a higher propulsive force is required to expel contents during the menstrual cycle. Uterine contractility increase could occur to overcome the increased frictional force with the passage of blood. Given the increased contraction, there will be an increased pain sensation. This explains the significant correlation of UCA with pain severity in patients with dysmenorrhea [23].
In adolescents with chronic pelvic pain, endometriosis has been reported in 20-73% of cases [24]. An appreciation for the clinical manifestations of endometriosis in the adolescent patient and careful history regarding adolescent dysmenorrhea may decrease the time from the onset of symptoms to presentation to diagnosis. The time from initial symptoms to diagnosis can be exceedingly long (mean 11.7 years in the U.S. and 8.0 years in the UK because of the variability of symptoms and signs and confusion with other disorders [25,26]. Early intervention can, ideally, decrease the long-term sequelae of this disease, such as chronic pain, dyspareunia, disruptions in social and occupational functioning, pelvic mass, and infertility, and improve the overall quality of life for women with this condition.
Physical findings suggest that endometriosis includes induration and tenderness of the posterior cul-de-sac uterosacral ligaments with tenderness/nodularity and a fixed, retroverted uterus. Propst and colleagues described reports of women with chronic pelvic pain with significant lateral displacement of most of the cervix to the left of the vaginal midline axis observed on vaginal speculum examination in patients with laparoscopically documented endometriosis [24]. As explained by Sahin et al.lateral displacement of the cervix is caused by the shortening of one of the cardinal ligaments that pull the cervix laterally [23]. What specifically elicits the tenderness is pushing the cervix towards the midline. The lateral retraction of the cervix is maximal when the ipsilateral uterosacral ligament is involved and contributes to increased pelvic pain, especially during menstruation, which is corroborated by Juang et al.’s case report in which there was a significant reduction in primary dysmenorrhea after using Laparoscopic Uterosacral Nerve Ablation (LUNA) [18,20]. Lateral cervical displacement is commonly associated with endometriosis; however, there have been cases of cervical displacement related to women with cervicitis. Gjoni & Muneyyirci-Delale explained that chronic cervicitis leads to the thickening and shortening of the uterosacral ligaments, leading to lateral removal of the cervix [26].
Further, Barbieri reported stenosis of the external os and its association with endometriosis in women with chronic pelvic pain [27]. Essentially, the many factors causing pain in patients with endometriosis, even cervicitis, involve cervical displacement due to scarring of the ipsilateral uterosacral ligament, and cervical stenosis is one of many common gynecologic findings that may be strongly associated with endometriosis in patients [20,24]. These observations have enabled clinicians to add a potentially new sign to the collection of physical findings related to endometriosis. But detailed comprehension of the pathophysiology and effective treatment of primary dysmenorrhea is still largely elusive.
Materials and Methods
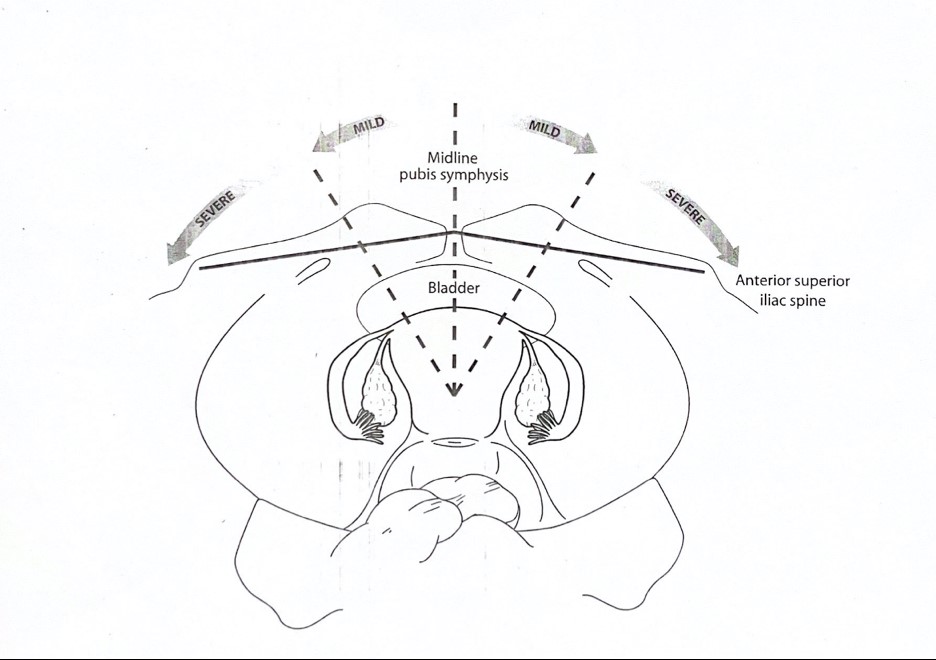
After Institutional Review Board approval, this retrospective chart review analyzed 1500 reproductive endocrine and infertility patient charts were reviewed for the presence of teenage dysmenorrhea, lifetime dysmenorrhea, and/or lateral displacement of the cervix and/or uterus at the State University of New York Downstate Health Sciences University. Of these, 323 patients were found to meet these criteria. Lateral cervical and uterine displacements were recorded as the extent of deviation from the midline pubic symphysis, and anterior/posterior deviation was designated as anteverted (anterior) or retroverted (posterior) based on pelvic examination.
Patient charts were reviewed for age, weight, height, BMI, number of pregnancies, number of live births, cycle regularity, dyspareunia, number of previous pelvic surgeries, number of previous cesarean sections, number of years of oral contraceptive use, number of years of Depo-Provera use, anteverted vs. retroverted uterus, presence of fibroids and their size, whether teenage dysmenorrhea was present and the severity, number of days of cramps, number of total years of dysmenorrhea, maximum severity of dysmenorrhea in the lifetime.
Dysmenorrhea was graded according to Biberoglu and Berhman criteria (0=no pain, 1= mild pain, 2=mild to moderate, 3=moderate, 4=moderate to severe, 5=severe) [22]. The average severity of dysmenorrhea in the teenage years (Tsev) and the maximum severity in the patient’s lifetime (maxsev) were noted separately. The data are presented as means +/- SE. P<0.05 was defined as statistically significant
Cervix Position: Normally, the cervix is a midline structure in direct alignment with the urethra and the anus [28]. When the cervix is displaced to the left or right of the midline, more than half of the cervix can be seen on visual inspection (while the remainder of the cervix is out of view due to its proximity to the lateral vaginal fornix); this is called mild cervical displacement. This is termed severe cervical displacement when less than half of the cervix can be seen on visual inspection. Displacement was confirmed with the bimanual examination and observed that one lateral fornix was wider than the other. With the examining hand in the double barrel pistol position, the cervix is palpated with the middle finger. When the external cervical os is not palpated in the midline using this method, the cervix is deemed to be laterally displaced.
Uterine Position: The nonpregnant uterus is situated midline in the pelvic cavity between the balder and rectum. The uterus is slightly anteflexed in 80% of women. When a female is standing upright, the uterus is horizontal and is partially flexed anteriorly, with the fundus resting upon the bladder. In the standing position, the cervix is directed back toward the top of the sacrum, with the external os quite close to the ischial spine. Some degree of uterine retroversion or retroflexion is observed in 20% of healthy women [29].
Arbitrarily, we introduced an imaginary line, midline, between the symphysis pubis and the anterior superior iliac spine. That line was then divided in half to differentiate between mild and severe uterine displacement. When the uterus was observed between the imaginary line and public symphysis, it was documented as mild uterine displacement. When the uterus was observed between the imaginary line and the anterior superior iliac spine, it was documented as severe uterine displacement (Figure 1).
Results
When looking at parameters such as the number of pregnancies and live births, number of pelvic surgeries, Cesarean sections, cervical surgeries, oral contraceptive pill use, and prior medical therapy for endometriosis, none were found to correlate with maxsev and Tsev. That is why these factors were not adjusted for in the analysis.
Results were obtained using factorial ANCOVA to assess the effects of lateral and anterior-posterior displacement of the uterus on Tsev and maxsev. When adjusting for age and BMI, lateral and anterior-posterior displacement of the uterus were insignificant. However, jointly, there was a significant interaction (p=0.002). Cervical displacement was not found to be significantly correlated with maxsev and Tsev. The degree of lateral uterine displacement did not seem to be related to the severity of dysmenorrhea; however, absolute lateral displacement of the uterus, combined with anterior-posterior displacement, played a significant role.
In patients with lateral displacement of the uterus, Tsev associated with a retroverted uterus was more significant than that associated with an anteverted uterus (4.24±0.24 vs. 3.35± 0.12, p=0.001, Fig 2 and 3). In the absence of lateral uterine displacement of the uterus, the anterior-posterior displacement did not affect Tsev (3.88+/-0.27 vs3.13+/-0.36, p=0.1). When maxsev is considered with the lateral displacement of uterus, the severity was greater in adult patients with retroverted compared to anteverted uteri (4.53±0.19 vs. 3.94±0.1, p=0.007, Figure 2 and 3). In the absence of lateral uterine displacement, anterior-posterior displacement did not influence the maximum severity of dysmenorrhea (3.99±0.21 vs. 3.60±0.29, p=0.3, Figure 2 and 3).
This is supported by the fact that prior pelvic surgeries, Cesarean sections, medications, and the number of live births did not affect the lateral displacement of the uterus, maxsev, or Tsev in our patient population.
Discussion
Lateral displacement of the cervix and its relation to endometriosis have been reported early [28,30]. Our data reveals no significant correlation between cervical displacement and the severity of menstrual pain. A correlation was found between the maximum severity of dysmenorrhea (maxsev) and teenage severity of dysmenorrhea with lateral displacement of the uterus (Tsev). When the parameters were studied alone, there was no significance of lateral or anterior-posterior displacement of the uterus on Tsev or maxsev. Patients with a retroverted uterus and lateral displacement of the uterus had significantly higher Tsev and maxsev than those with anteverted uteri and lateral displacement. RL Barbieri reported stenosis of the external os and its association with endometriosis in women with chronic pelvic pain. [18]. Possibly, the retroverted and laterally displaced uterus causes significantly more pain because of an outlet obstruction to menstrual flow due to endocervical canal narrowing that causes more retrograde menstruation and adhesion formation in the abdominal cavity (Figure 4). In Sahin et al., though the precise definition of uterine cervical angle and its impact on dysmenorrhea was discussed in detail, there was no manual examination of the specific lateral, anterior/posterior displacement of the uterus, which also plays a role in dysmenorrhea [23]. Endometriosis was visually documented at the surgery for 24 of 25 women with chronic pelvic pain and stenosis of the external cervical os [18]. Lateral displacement is caused by the shortening and retraction of one cardinal ligament [24]. When the lateral retraction is maximal, the ipsilateral uterosacral ligament may be involved with nodular endometriosis [24]. We had limited patients who had nodular uterosacral ligaments. We hypothesize that the lateral displacement of the uterus is caused, to begin with, by the non-symmetric distribution of endometrial implants from retrograde menstrual flow in these patients, and this leads to the vicious cycle of adhesion formation, distortion of the anatomy, further outlet obstruction, greater retrograde menstruation, further adhesions formation, more significant distortion of pelvic anatomy, and so on (Figure 4). This is supported by the fact that prior pelvic surgeries, cesarean sections, medications, and several live births did not affect the lateral displacement of the uterus, maxsev or Tsev in our patient population.
Further, Iwata et al. completed a systematic review/network meta-analysis exhibiting how progestins are more effective than low estrogen-progestin in improving pain relief [37]. In addition, alternative treatments are also encouraged, such as applying topical heat, acupuncture/electro-acupuncture, and even transcutaneous electrical nerve stimulation (TENS) is shown to provide significant relief in pain symptoms [38]. For women who have severe symptoms such that they do not respond to the therapies listed above, GnRH agonist with “add-back” hormonal treatment such as norethindrone acetate (as the first line) with limits of one year or GnRH beyond six months; if women continue to be refractory, then aromatase inhibitors can be added [39]. Taylor et al. provided evidence that higher and lower doses of Elagolix (GnRH antagonist) improved both dysmenorrhea and endometriosis-linked lower abdominal pain [40]. Surrey et al. proved that consistent use of Elagolix reduced dysmenorrhea, dyspareunia, and even non-menstrual pelvic pain [41]. Linzagolix is an alternative GnRH antagonist with current positive phase 3 results. There is evidence that it can decrease the uterus’ adenomyotic volume, improve pelvic pain and dysmenorrhea, and enhance the quality of life [42]. Lastly, once-a-day Relugolix(GnRH antagonist), in combination with progestin and estradiol, significantly improved pain linked with endometriosis [43]. Notably, the GnRH agonists and antagonists are suggested second-line treatments due to associated side effect profiles (such as decreasing bone density). With women who continue to have endometriosis-associated pain that is refractory to the medical treatments, aromatase inhibitors can be recommended and may be prescribed in combination with oral contraceptives, progestins, GnRH agonists/GnRH antagonists. Danazol is not recommended due to unwanted side effects (acne, vaginal spotting, weight gain, muscle cramps, voice deepening). In summary, the medications that lead to a significant clinical reduction in pain include progestins, anti-progestins, combined OCPs, Gonadotropin agonists (GnRH), GnRH antagonists, danazol, aromatase inhibitors [35].
In fact, for patients with primary dysmenorrhea there may be no associated physical findings and pelvic examination is usually normal. However, findings that are suggestive of dysmenorrhea include uterosacral ligament abnormalities such as nodularity, thickening, or focal tenderness; lateral displacement of the cervix due to asymmetric involvement of one of the uterosacral ligaments via endometriosis, and cervical stenosis. Rationale for why uterosacral ligament abnormalities can lead to dysmenorrhea remains largely unclear; however, Whiteside and Falcone propose that this location is richly innervated with nerves and locations of uterosacral invasion have been shown to increase expression of NGF (nerve growth factor) which is associated with increased pain [31] . Additional rationale for dysmenorrhea in women includes abnormal restriction of lumbosacral vertebrae movement leading to increases in body fluid within the pelvis and contraction of the uterus, worsening menstrual pain through excessive prostaglandin secretion [32]. Per Proctor et al., in women with dysmenorrhea, spinal alignment procedures led to alleviation of these symptoms [32]. Uterine and cervical anatomy play a major role in contributing to severity of primary dysmenorrhea; in fact, Zebitay et al. found that the length of cervical longitudinal and transverse axis and uterine cervical volume significantly contribute to this outcome [33]. Cagnacci et al. provided evidence that the greater the uterine flexion angle, the stronger that the myometrial contraction has to be in order to overcome the inner cervical orifice to eject menstrual contents [34]. Thus, dysmenorrhea is worsened [34].
Our findings suggest the need for serious attention to adolescent patients who complain of dysmenorrhea and those with cervical or uterine displacement on physical examination. A detailed history regarding the severity of pain should be investigated. Many adolescents are offered NSAIDs or oral contraceptive medication as an initial approach to any level of dysmenorrhea. Per the European Society of Human Reproduction and Embryology (ESHRE) guidelines for treating endometriosis-associated pain, the use of NSAID analgesics is a weak recommendation for treating dysmenorrhea in relation to endometriosis [35]. Also, in the 323 patients studied, oral contraceptive pill use was not found to affect the severity of pain nor the displacement of the anatomy. However, we only recorded the length of time or oral contraceptive pill use, not the length of time the patient experienced dysmenorrhea before the oral contraceptive pill was initiated. We cannot determine whether the damage appeared before the onset of oral contraceptive pill use or regardless of its use. Continuous use of the OCPs effectively reduces endometriosis-associated endometrial pain.
ESHRE recommends using medications that alter the hormonal atmosphere by decreasing ovarian activity or acting on hormonal receptors [35]. Further, Donnez et al. provided evidence that the oral progestin-only treatment for pelvic pain is more effective than OCPs as first-line therapy for treating pelvic pain associated with endometriosis and even decreases the anatomic extent of endometrioid lesions [36]. Per Donnez et al., there is no clinical evidence of combined OCP use to alleviate pelvic pain associated with endometriosis [36].
Suppose the treatments above or laparoscopic ablation have not worked well. More invasive treatments such as laparoscopic uterosacral ablation (LUNA), adjuvant presacral neurectomy (PSN), or a nerve block procedure must be considered. PSN could be considered for women with consistent pelvic pain for conservative surgical management; LUNA is not recommended as there is no significant improvement in pain [44]. As a last resort, total abdominal hysterectomy and bilateral salpingo-oophorectomy could be considered in women who do not desire to take the medications mentioned above/medications that are not effective for them and no longer wish to conceive [45-48]. When surgery is considered, surgeons can consider excision instead of ablation of endometriosis to reduce endometriosis-associated pain. However, surgical management is unsuitable for young women as they may wish to preserve their fertility [35]. Interesting to alternative to surgical management include vaginal delivery and cervical dilatation. Juang et al.’s prospective study provided evidence that severity of dysmenorrhea improved after first spontaneous vaginal delivery as the birth changes the UCA and reduces cervical canal resistance [49]. Additionally, Dawood provided evidence that cervical dilatation in patients with primary dysmenorrhea improved their symptoms as there was a reduction of resistance to cervical excretion which leads to a decrease in prostaglandin release and uterine pressure [50]. Treating dysmenorrhea early in the patient’s life is crucial to avoid harmful sequelae such as hyperalgesic priming through central nervous system reorganizing (predisposing patients to chronic pelvic pain), and can negatively impact mental health of adolescents [51].
Conclusion
The severity of pain associated with a retroverted uterus in relation to an anteverted uterus depends on whether the uterus is also displaced laterally. With the lateral displacement of the uterus or cervix or the combination of the two, an outflow obstruction might occur and result in retrograde menstruation. The distortion of the anatomy may stem from asymmetric endometriotic involvement of one uterosacral ligament causing it to shorten. The anatomic distortion may be due to prior surgery, infection, or anomaly. We propose that the result is the same: retrograde menstruation and the resulting outflow obstruction from the anatomic distortion cause more retrograde menstruation. The study highlights the importance of correct positioning of the uterus in dysmenorrhea management
Regarding recommended management, using oral contraceptive pills may decrease endometrial thickness, and using NSAIDs may decrease prostaglandins, reducing retrograde menstruation and dysmenorrhea. Most interestingly, in a reproductive age group, cervical dilation due to vaginal delivery can decrease pain and the occurrence of endometriosis. Further, if patients undergo laparoscopic endometrial ablation, the cervix should be dilated to decrease dysmenorrhea and reduce the change of retrograde menstruation [52]. Nevertheless, it is essential to recognize this process early in the patient’s life to avoid harmful sequelae as much as possible.
This study is a retrospective chart review; thus, recall bias could be introduced. Recruiting patients from multiple centers to increase diversity of patient population and minimize possible selection bias would be a point of expansion for this project. Likewise, demographic characteristics and quality of patient lives were not evaluated in our data collection which can be included in future studies to examine any differences in the clinical presentation of dysmenorrhea and prevalence across different ethnic groups. Further, collecting data on menstrual cycle patterns such as cycle length and regularity, as these factors could influence dysmenorrhea severity. As objective measures were use in this study, the use of ultrasonography or MRI should be implemented for more accurate measurements. Regarding management of dysmenorrhea, clinical trials to evaluate the effectiveness of different treatment options (nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapies, or surgical interventions). Further studies to view the relationship between cervical displacement and dysmenorrhea can be prospective where selected patients are followed up over a concrete period of time, to yield more robust data. Since this study only viewed the impact of dysmenorrhea severity in patients with lateral displacement of the uterus and cervix, further studies could investigate impact of other variables on dysmenorrhea severity stratified by other variables such as patient age, BMI, and contraceptive use.
- French L (2005) Dysmenorrhea. Am Family Physician 71: 285-91
- Davis AR, Westhoff CI (2001) Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol 14: 3-8.
- De Sanctis V, Soliman A, Bernasconi S, Bianchin L, Bona G et al. (2015) Primary Dysmenorrhea in Adolescents: Prevalence, Impact, and Recent Knowledge. Pediatri Endocrinol Rev 13: 512-20.
- Dawood M Yusoff (1990) Dysmenorrhea. Clin Obstet Gynecol 33.
- Campbell MA, McGrath PJ (1997) Use of medication by adolescents for the management of menstrual discomfort. Arch of Ped Adolesc Med 151: 905.
- Sachedina A, Todd N (2020) Dysmenorrhea, endometriosis and chronic pelvic pain in adolescents. J Clin Res Pediatr Endocrinol 12: 7-17.
- Wilson CA, Keye WR Jr (1989) A survey of adolescent dysmenorrhea and premenstrual syndrome frequency. A model program for prevention, detection, and treatment. J of Adolesc Healthcare 10: 317.
- ACOG (2008) Committee Opinion No. 760: Dysmenorrhea and Endometriosis in the Adolescent. Obstet Gynecol 132: 249-58.
- Sahin N, Kasap B, Kirli U, Yeniceri N, Topal Y (2018) Assessment of anxiety-depression levels and perceptions of quality of life in adolescents with dysmenorrhea. Reproductive health 15: 1-7.
- Akin MD, Weingand KW, Hengehold DA, Goodale MB, Hinkle RT, Smith RP (2001) Continuous low-level topical heat in the treatment of dysmenorrhea. Obstetrics & Gynecology 97: 343-9.
- Thomas M, Lundeberg T, Björk G, Lundström Lindstedt V (1995) Pain and discomfort in primary dysmenorrhea is reduced by preemptive acupuncture or low frequency TENS. European journal of physical medicine & rehabilitation 5: 71-6.
- Gokhale LB (1996) Curative treatment of primary (spasmodic) dysmenorrhoea. The Indian journal of medical research 103: 227-31.
- Matthewman G, Lee A, Kaur JG, Daley AJ (2018) Physical activity for primary dysmenorrhea: a systematic review and meta-analysis of randomized controlled trials. American journal of obstetrics and gynecology 219: 255-e1.
- Dawood MY (1981) Hormones, prostaglandin, and dysmenorrhea. In: Dawood MY, editor. Dysmenorrhea. Baltimore (MD): Williams and Wilkins 20-52
- Palomba S, Russo T, Falbo A et al. (2006) Laparoscopic uterine nerve ablation versus vaginal uterosacral ligament resection in postmenopausal women with intractable midline chronic pelvic pain: A randomized study. Eur J Obstet Gynecol Reprod Biol 129: 84-91
- Pulkkinen MO (1983) Prostaglandins and the non-pregnant uterus. The pathophysiology of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl 113: 63-7.
- Clemenza S, Vannuccini S, Capezzuouli T, Meleca CI, Pampaloni F, Petraglia F (2021) Is primary dysmenorrhea a precursor of future endometriosis development? Gynecol Endocrinol 37: 287-93.
- Clemenza S, Vannuccini S, Capezzuouli T, Meleca CI, Pampaloni F, Petraglia F (2021) Is primary dysmenorrhea a precursor of future endometriosis development? Gynecol Endocrinol 37: 287-93.
- Howard FM (2009) Endometriosis and mechanisms of pelvic pain. Journal of minimally invasive gynecology 16: 540-50.
- Bulletti C, De Ziegler D, Polli V, Del Ferro E, Palini S, Flamigni C (2002) Characteristics of uterine contractility during menses in women with mild to moderate endometriosis. Fertile Steril 77: 1156-61.
- Harada T (2013) Dysmenorrhea and endometriosis in young women. Yonago acta medica 56: 81.
- M Dziadosz, TA Bennett, C Dolin et al. (2016) “Uterocervical angle: a novel ultrasound screening tool to predict spontaneous preterm birth,” American Journal of Obstetrics and Gynecology 215: 376-6.
- Sahin ME, Sahin E, Madendag Y, Madendag IC, Tayyar AT et al. (2018) The effect of anterior uterocervical angle on primary dysmenorrhea and disease severity. Pain Research and Management.
- Attaren M, Gidivani G (2003) Adolescent endometriosis. Obstet Gynecol Clin N Amer 30: 370-90.
- Giudice LC, Lee K (2004) Endometriosis. Lancet 364: 1789-99.
- Gjoni I, Muneyyirci-Delale O (2012) Cervicitis is associated with lateral cervical displacement. Medical Hypotheses 78: 134-5.
- Barbieri RL. Stenosis of the external cervical os: an association with endometriosis in women with chronic pelvic pain.
- Obstetrics and Gynecology, Fourth Edition. J Robert Wilson, Clayton T. Beechmar, Elsie Reid Carrington (1987) The CV Mosby Company, St. Louis 286-5.
- Williams Obstetrics, 14th Edition. Louis Hellman, Jack A Pritchard. Appleton-Century- Crofts, New York, 1971.
- Biberoglu KO, Behrman SJ (1981) Dosage aspects of danazol therapy in endometriosis: short-term and long-term effectiveness. Am J Obstet Gynecol 137: 645-54.
- Whiteside JL, Falcone T (2003) Endometriosis-related pelvic pain: what is the evidence? Clinical obstetrics and gynecology 46: 824-30.
- Proctor ML, Hing W, Johnson TC et al. (2006) Spinal manipulation for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 19: CD002119.
- Proctor ML, Hing W, Johnson TC et al. (2006) Spinal manipulation for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 19: CD002119.
- Zebitay AG, Verit FF, Sakar MN, Keskin S, Cetin O, Ulusoy AI (2016) Importance of cervical length in dysmenorrhoea aetiology. Journal of Obstetrics and Gynaecology 36: 540-3.
- Cagnacci A, Grandi G, Cannoletta M, Xholli A, Piacenti I, Volpe A (2014) Intensity of menstrual pain and estimated angle of uterine flexion. Acta obstetricia et gynecologica Scandinavica, 93: 58-63.
- Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F (2022) ESHRE Endometriosis Guideline Group, ESHRE guideline: endometriosis, Human Reproduction Open 2: hoac009,
- Donnez J, Dolmans MM (2021) Endometriosis and medical therapy: from progestogens to progesterone resistance to GnRH antagonists: a review. Journal of Clinical Medicine 10: 1085.
- Donnez J, Dolmans MM (2021) Endometriosis and medical therapy: from progestogens to progesterone resistance to GnRH antagonists: a review. Journal of Clinical Medicine 10: 1085.
- Iwata M, Oikawa Y, Shimizu Y, Sakashita N, Shoji A (2022) Efficacy of Low-Dose Estrogen-Progestins and Progestins in Japanese Women with Dysmenorrhea: A Systematic Review and Network Meta-analysis. Advances in Therapy 1-18.
- Woo HL, Ji HR, Pak YK, Lee H, Heo SJ (2018) The efficacy and safety of acupuncture in women with primary dysmenorrhea: a systematic review and meta-analysis. Medicine 97.
- Zupi E, Marconi D, Sbracia M et al. (2004) Add-back therapy in the treatment of endometriosis-associated pain. Fertil Steril 82: 1303.
- Taylor HS, Giudice LC, Lessey BA, Abrao MS, Kotarski J (2017) Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. New England Journal of Medicine 377: 28-40.
- Surrey E, Taylor HS, Giudice L, Lessey BA, Abrao MS (2018) Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstetrics & Gynecology, 132: 147-60.
- Donnez J, Taylor HS, Taylor RN, Akin MD, Tatarchuk TF (2020) Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone–antagonist: a randomized clinical trial. Fertility and Sterility 114: 44-55.
- Giudice LC, As-Sanie S, Ferreira JCA, Becker CM, Abrao MS (2022) Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate phase 3, randomised, double-blind studies (SPIRIT 1 and 2). The Lancet 399: 2267-79.
- Taylor HS, Seli EU, Pal L (2020) Speroff’s clinical gynecologic endocrinology and infertility. Wolters Kluwer.
- Cosson M, Querleu D, Donnez J et al. (2022) Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: results of a pro- spective, multicenter, randomized study. Fertil Steril 77: 684-92.
- Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG (2003) Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for asymptomatic endometriosis: a pilot study. Fertil Steril 80: 305-9.
- Carlson KJ, Miller BA (1994) Fowler FJ Jr. The Maine Women’s Health Study: I. Outcomes of hysterectomy. Obstet Gynecol 83: 556-65.
- . Nasir L, Bope ET (2004) Management of pelvic pain from dysmenorrhea or endometriosis. The Journal of the American Board of Family Practice 17: 43-7.
- Juang CM, Yen MS, Twu NF, Horng HC, Yu HC, Chen CY (2006) Impact of pregnancy on primary dysmenorrhea. International Journal of Gynaecology and Obstetrics 92: 221-7.
- Dawood MY (2006) Primary dysmenorrhea: advances in pathogenesis and management. Obstetrics and Gynecology 108: 428-41.
- MacGregor B, Allaire C, Bedaiwy MA, Yong PJ, Bougie O (2023) Disease Burden of Dysmenorrhea: Impact on Life Course Potential. International Journal of Women's Health 499-509.
- Bulletti C, Montini A, Setti PL, Palagiano A, Ubaldi F, Borini A (2010) Vaginal parturition decreases the recurrence of endometriosis. Fertility and sterility 94: 850-5.


Figures at a glance