Microscopical Diagnosis of Bacterial Vaginosis Compared with Two Different Molecular Methods
Received Date: May 11, 2024 Accepted Date: June 11, 2024 Published Date: June 14, 2024
doi: 10.17303/jwhg.2024.11.103
Citation: Georgios Charonis, Klara Törneke, Iris Vikström, Malin Farnebäck, Anna Ansell-Schultz, et al. (2024) Microscopical Diagnosis of Bacterial Vaginosis Compared with Two Different Molecular Methods. J Womens Health Gyn 11: 1-14
Abstract
Background: Consensus has not yet been reached on the optimal method for diagnosing bacterial vaginosis. As methods currently used in clinical routine diagnostics are subjective, there is a need for other objective methods. Different molecular diagnostic methods for the diagnosis of bacterial vaginosis have been introduced as alternatives to traditional clinical diagnostic methods.
Materials and Methods: A total of 243 women attending local youth clinics in Skaraborg county of Sweden for STI screening or malodorous discharge were recruited. To clinically diagnose BV, airdried vaginal samples were evaluated microscopically by using the modified Hay/Ison criteria. The result of the evaluation was divided into normal, intermediate, or BV vaginal flora. In this study, the intermediate group was considered as BV negative when compared with the Aptima BV assay and Dynamic Code's BV and Candida test.
The Aptima BV assay which is a real-time transcription-mediated amplification (TMA) analysis and detects and quantitates rRNA from Lactobacillus spp. (L. gasseri, L. crispatus, and L. jensenii), G. vaginalis, and A. vaginae. The assay uses an algorithm to report a qualitative result for bacterial vaginosis based on detection of the included microorganisms.
Dynamic Code's BV and Candida test uses a different algorithm and analyzed multiple BV associated bacteria, i.e., G., vaginalis, A. vaginae, BVAB2, Sneathia spp., Megasphaera spp., and Mobiluncus spp. These bacteria are related to the number of lactobacilli and combined into BV positive or BV negative.
Patients diagnosed with BV were offered treatment with dequalinium chloride vaginal tablets (Fluomizin®. In Nordic countries Donaxyl®), one vaginal tablet for 6 days, followed by treatment with 2% clindamycin vaginal cream (Dalacin ®), administered daily for 7 days. Treated patients were asked to collect a new self-collected vaginal sample one to two months after completed treatment.
Results: Bacterial vaginosis was diagnosed in 65% of the vaginal samples and the cure rate after treatment with dequalinium chloride vaginal tablets/clindamycin vaginal cream was 82% after 2 months. The kappa index between the routine clinical diagnosis and the molecular assays were 0.75 for Aptima BV Assay and 0.73 for Dynamic Code. All samples with discrepancies between the routine clinical diagnosis and the molecular assays were reexamined and photographed. After re-examination of the photographs, the primary clinical diagnosis remained unchanged in 32 cases. However, for 16 BV cases the primary clinical diagnoses were changed to normal or intermediate vaginal flora and for 11 cases the diagnoses were changed from normal flora to BV. These evaluations were performed blinded to the results of the molecular assays. After reevaluation, the kappa index between the clinical diagnosis and molecular assays were 0.82 for Aptima BV Assay and 0.89 for Dynamic Code. In addition, for “test of cure”, the kappa index was 0.54 and 0.60, respectively. Was no statistical difference between the two molecular assays.
Conclusions: The kappa index shows that the molecular assays are adequate in diagnosing BV. Notably, as a “test of cure” the usability of the molecular assays is only moderate. However, this can depend on the small number of patients included in the study and further investigations are necessary. Interestingly, in most cases where microscopy showed an intermediate vaginal flora, the result of the molecular assay was BV positive. Thus, in future investigations, the questions whether intermediate vaginal flora is transient or constant and if molecular assays may discover relapses earlier than microscopic evaluation should be addressed. Trial registration: Clinical trial registration 14 August 2019 # NCT04047482, retrospectively registered.
Keywords: Bacterial Vaginosis; Vaginitis; Dequalinium Chloride Treatment; Clindamycin Treatment; Polymerase Chain Reaction (PCR); Molecular Diagnostic Techniques; Test of Cure; Hay/Ison
Introduction
Bacterial vaginosis (BV) is a common cause of vaginal inflammation and discomfort in women of reproductive age. Although no single etiological factor has been identified, molecular studies have shown that a change in the vaginal microbiota is associated with BV infection [1,2]. The vaginal microbiome is a dynamic ecosystem and includes more than 200 bacterial species. Throughout a woman’s life, the microbiome is exposed and affected by several stressors, such as hormones and habits. These modifications of the vaginal microenvironment can dramatically change the microbiome composition, resulting, for example, in the depletion of Lactobacillus spp., reduction of microbial diversity and occurrence of dysbiosis e.g., BV [3].
BV is commonly clinically diagnosed with Amsel’scriteria [4]. According to Amsel’s criteria, three out of four clinical criteria should be fulfilled to confirm the diagnosis. These criteria include typical homogeneous vaginal discharge, an elevated vaginal pH >4.5, a positive potassium hydroxide test (whiff test), and the presence of clue cells. These criteria can be considered subjective, and their interpretation can differ among clinicians. Across different studies, there has also been a difference in the cutoff value used for clue cells. Eschenbach et al. (5) concluded that most BV-infected patients possess more than 20% clue cells; therefore, this cutoff value has been used in several treatment research studies [6] to determine a BV diagnosis. Hence, to fulfill the diagnosis of BV, the patient must have more than 20% clue cells. Consequently, women with less than 20% clue cells could be considered cured of their BV infection, which is not consistent with the original works by both Amsel [4] and Gardner [7] where the term “clue cells” was originally introduced.
The most accepted laboratory diagnostic test for BV is Nugent’s criterium [8]. A swab is obtained from the lateral vaginal wall and is rolled onto a glass slide. The smears are subsequently Gram stained. The number of Lactobacillus spp. morphotypes is calculated; whereby the presence of more than 30 lactobacilli per vision field give a score of 0, 5–30 lactobacilli give a score of 1, 1–4 lactobacilli gives a score of 2, an average score of less than 1 lactobacilli gives a score of 3, and no lactobacilli observed per visual field gives a score of 4. Gardnerella-like bacteria are scored in a similar manner but in the reverse order. In addition, the presence of curved gram-variable rods (Mobiluncus spp. morphotypes) will result in the addition of 1–2 points to the score depending on the number observed. A total score of 0–3 is considered normal vaginal flora, a score of 4–6 is considered intermediate, and a score of 7–10 is considered a conclusive diagnosis of BV. The use of a different microscope with an area that is 300% larger should require three times more bacteria, i.e., a total of 90 Gardnerella spp. morphotypes to achieve the same score as with a microscope with smaller area [9].
A simpler method was described by Hay et al. [10,11] in which the vaginal flora is divided into the following categories: normal (lactobacilli morphotypes—few Gardnerella spp. morphotypes), intermediate (equal numbers of lactobacilli and Gardnerella spp. morphotypes), and BV (few lactobacilli and many Gardnerella spp. morphotypes). The Hay/Ison criteria were originally developed using Gram-stained smears by oil immersion at a magnification of 1000x; however, because these criteria assess the type of flora and do not indicate the number of individual bacteria, as in Nugent’s criteria, it is possible to use these criteria with non-stained smears at relatively low magnification. A modification of the Hay/Ison criteria has recently been validated [12], where vaginal samples were first air-dried and upon arrival to the laboratory, rehydrated with saline before microscopic analyses. Thus, this allows the samples to be transported and saved for later investigation without affecting the sample’s integrity.
All above-mentioned diagnostic methods are based on subjective assessment and require highly skilled personnel, thus, there is a growing need to validate easier and objective diagnostic methods. Different molecular methods for the diagnosis of BV have been introduced [1,13,15]. Schwebke et. al. [15] published a molecular diagnostic method using the BD MAX™ Vaginal Panel from Becton, Dickinson and Company, which showed promising results, with the sensitivity 90.5% and specificity 85.8%.
Hologic [16] published their method where they relate the BV associated bacteria, G. vaginalis and A. vaginae, to Lactobacillus spp. with good results, sensitivity 84% and specificity of 86%. Previously, we have published a study [17] were we used Dynamic Code's method for BV. This method can analyze multiple BV associated bacteria, i.e., G. vaginalis, A. vaginae, BVAB2, Sneathia spp., Megasphaera spp., and Mobiluncus spp. These bacteria are also related to the number of Lactobacillus spp. and a sensitivity of 91% and specificity of 97% was obtained.
Numerous studies investigating the treatment of BV have been published [18,19], but there is still no consensus on how treatment should be evaluated. A considerable problem is that the “test of cure” is carried out at different timepoints, ranging from the day after, up to 3 weeks after the completion of treatment, or after the following menstruation. In this population-based study of women attending local maternity and youth clinics in Skaraborg county, Sweden, for STI screening or malodorous discharge, we investigated the usability of molecular diagnostics for bacterial vaginosis compared to routine clinical diagnosis using the modified Hay/Ison criteria. In addition, to this date, there is no consensus on when to perform a “test of cure” after treatment completion, thus, we also investigated the performance of molecular methods as a “test of cure”.
The primary goal of this study was to investigate the Kappa index of two different molecular methods for the diagnosis of BV compared to the microscopical diagnosis using the modified Hay/Ison criteria.
The second goal was to treat BV, first with dequalinium chloride vaginal tablets and directly after continued treatment with clindamycin cream for 7 days, and study if the molecular test can be used as “test of cure”.
Materials and Methods
Study Population
The patients were recruited from local maternity and youth clinics in Skaraborg county, Sweden. Eligibility for inclusion in the study was based on the following criteria: menstruating women from 15 years of age, using any contraceptive, not planning to get pregnant, experiencing malodorous discharge, and testing negative for Chlamydia trachomatis and Neisseria gonorrhoeae with a standard laboratory test. Women who have undergone any other antibiotic treatment during the last month before or during the commencement of the study’s treatment were excluded
Sample Collection
Three vaginal samples were collected from women presenting with symptoms of malodorous vaginal discharge either by a midwife or by the woman herself. Each sample was collected from the lateral fornix. The first sample was transferred onto a microscope slide, air-dried, and sent to Skaraborgs Hospital’s Gynaecology Department in Skövde, Sweden. The second sample, collected using a FLOQSwabs™ (Copan, Brescia, Italy), was sent to Dynamic Code (Linköping, Sweden) for analysis of BV (Test for Bacterial vaginosis and Candidiasis). The third sample was collected using the Aptima Multitest Swab Specimen Collection Kit (Hologic, Inc. San Diego, CA, USA) and sent to our regional laboratory for analysis of C. trachomatis and N. gonorrhoeae (Aptima Combo 2® assay for CT/NG, Hologic Inc.) and BV (Aptima® BV Assay, Hologic Inc.).
Clinical Diagnosis of BV
The microscope slide was examined after adding saline using a phase contrast microscope (Zeiss double ocular) at a magnification of 400x according to the modified Hay/Ison criteria for the diagnosis of BV [12]. The vaginal flora was divided into the following three categories depending on the relative number of Lactobacillus spp. morphotypes compared to Gardnerella spp. morphotypes: normal (grade 1), intermediate (grade 2), and BV (grade 3). At least four fields were evaluated in all vaginal samples. In comparison with the molecular diagnostic methods, the intermediate group was considered as BV negative.
All samples with discrepant results between the microscopic evaluation and the molecular methods was reexamined blinded to the previous diagnosis or molecular diagnosis. Some slides were also photographed to enable both researchers to examine the same image (IW and PGL).
Treatment
Patients diagnosed with BV were offered treatment with dequalinium chloride vaginal tablets (Fluomizin® or in Nordic countries Donaxyl®) followed by treatment with 2% clindamycin vaginal cream (Dalacin ®). The 13 days treatment scheme was one dequalinium chloride tablet for 6 days and thereafter daily administration of clindamycin for 7 days.
Treated patients were asked to provide new self-- collected vaginal samples between one and two months after treatment completion. One for microscopy and 2 for molecular tests. If vaginal samples were provided after more than 12 months, this was considered a new test. If the microscopic evaluation of this new vaginal sample (“test of cure”) showed persisting BV, the patient was offered the same treatment regime again. A subset of patients was also tested a third and a fourth time.
Hologic (Aptima BV Assay)
This assay utilizes real-time transcription-mediated amplification (TMA) and detects and discriminates RNA markers from the Lactobacillus spp. (L. gasseri, L. crispatus and L. jensenii), and from G. vaginalis, and A. vaginae [15]. The assay uses an algorithm to report a qualitative result for bacterial vaginosis based on detection of target organisms. The algorithm compares signal emergence times for each target organism to calibration information to determine the bacterial vaginosis Positive or Negative status of each sample.
Dynamic Code (Test for Bacterial vaginosis and Candidiasis)
The analysis is based on qPCR detection of A. vaginae, BVAB2, G. vaginalis, Megasphaera spp., Mobiluncus spp., Sneathia spp., Lactobacillus spp. The signal from each BV associated bacteria is related to the signal from Lactobacillus spp. and converted into a likelihood for BV. The results are reported qualitatively as positive or negative for BV. For vulvovaginal candidiasis (VVC) analysis, the fungal species C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis are detected and qualitatively reported as positive or negative for VVC.
The study was approved by the Regional Ethical Review Board (EPN) in Gothenburg (Dnr 2020- 01542)). Informed consent was obtained from all participants before participation. Clinical trial registration # NCT04047482, retrospectively registered.
Statistics
Kappa index and sensitivity and specificity was calculated using openepi.com, https://www.openepi.com/Diag nosticTest/DiagnosticTest.html
Results
From September 2020 until April 2022, a total of 243 women were recruited. The mean ages were 22 years,ranging from 15-26.
A total of 301 samples were analyzed using the Aptima BV Assay and 203 samples were analyzed using the Dynamic Code’s assay. Seven samples obtained an invalid result using the Aptima BV Assay and three using the Dynamic Code’s assay. In addition, two microscopic slides were not possible to evaluate. Thus, in total, 292 Aptima BV Assay samples and 198 Dynamic Code samples were included. Of these, 192 samples were analyzed with both assays. In total, 323 microscopic evaluations were done as some participants did only send in the microscopic slide as a test of cure.
The microscopic evaluation on the first sampling occasion resulted in 156 patients diagnosed with BV and 82 patients with negative for BV, giving a prevalence of BV of 65% in this cohort. The kappa index for diagnosis were 0.75 (0.66-0.84) n=233 (table 1) for the Aptima vs modified Hay/Ison and 0.73 (0.61-0.85) n=149 (table 2) for the Dynamic Code vs modified Hay/Ison.
Of the 156 treated women, only 70 women collected samples for “test of cure”. However, only 55 women collected a complete set of samples (including two samples for PCR analysis). Most samples were collected within 1-3 months (75%) after treatment completion. In 5 cases, samples were, however, collected after more than 6 months. Of the 70 reevaluated patients, 58 patients were cured from BV, resulting in a cure rate of 82.8%.
The kappa index for the “test of cure” was 0.39 (0.12-0.67), n=55 (table 3) for Aptima vs modified Hay/Ison and 0.36 (0.03-0.75) n=49 (table 4) for Dynamic Code vs modified Hay/Ison.
For all samples with discrepant results between the microscopic evaluation and the molecular methods, 40 samples with Aptima BV Assay and 28 with Dynamic Code the microscopic slides were reexamined. Some of the above samples were from the same patient, thus, 47 slides were reexamined. Of these, 20 samples they were the “test of cure” samples.
After re-examination, the primary diagnosis remained unchanged in 32 cases, of these it was 13 BV and 13 intermediate and 6 normal.
In 17 cases, the BV diagnosis was changed to negative and in 8 cases the negative was changed to positive for BV.
A new kappa index for diagnosis was calculated after re-examination, resulting in 0.82 (0.74-0.90) n=233 (table 5) for Aptima vs modified Hay/Ison and 0.89 (0.81-0.97) n=149 (table 6) for Dynamic Code vs modified Hay/Ison.
Similarly, a new kappa index for “test of cure” was calculated to 0.54 (0.29-0.79) n=55 (table 7) for Aptima vs modified Hay/Ison and 0.60 (0.26-0.94) n=44 (table 8) for Dynamic Code vs Hay/Ison. No statistical difference between the two molecular methods for diagnosing BV could be detected. The kappa index comparing the two molecular methods was 0.69 (0.59-79), n=192, data not shown.
When analyzing the discrepancies between the methods, it was noted that when samples were negative with the Aptima BV Assay in patients diagnosed with BV, only one slide showed a typical
BV Vaginal Flora
When the samples were positive with the Aptima BV Assay in women not diagnosed with BV, the samples showed an intermediate vaginal flora according to Hay/Ison. It was also common with a high prevalence of Candida spp. and white blood cells (WBC) on these slides. When the Dynamic Code assay showed negative results in samples for positive for BV, it was either because of simultaneous Candida spp. infection or thin sample preparation. In addition, some of these slides displayed very short bacteria morphotypes, making it difficult to distinguish between lactobacilli and anaerobic bacteria. Hence, making a correct diagnosis was complicated in these cases.
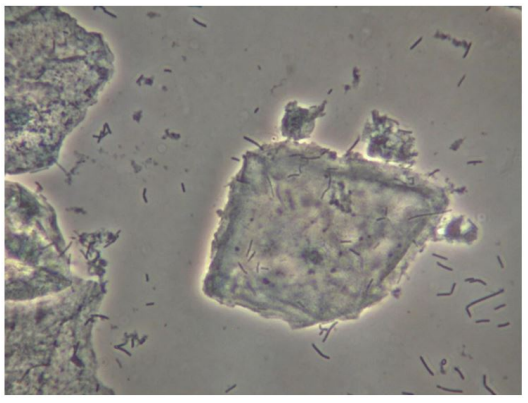
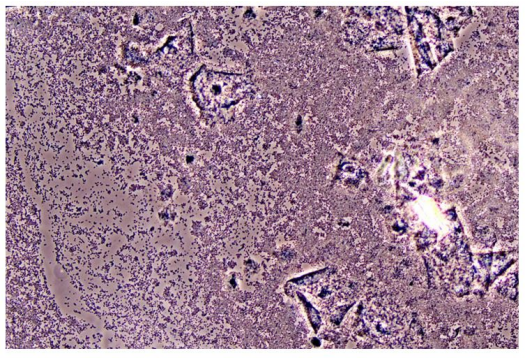
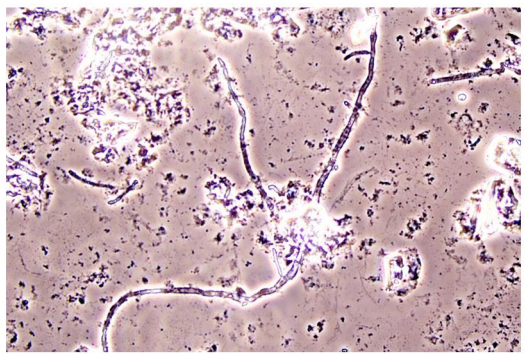
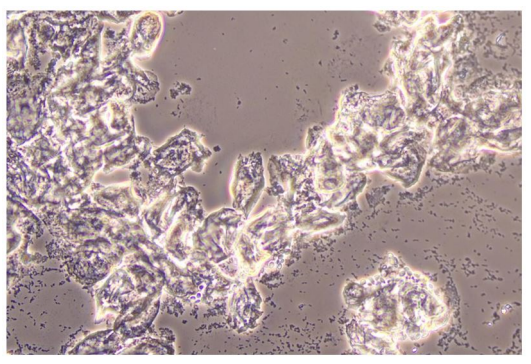
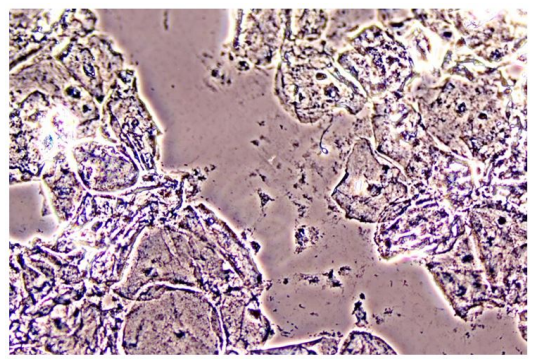
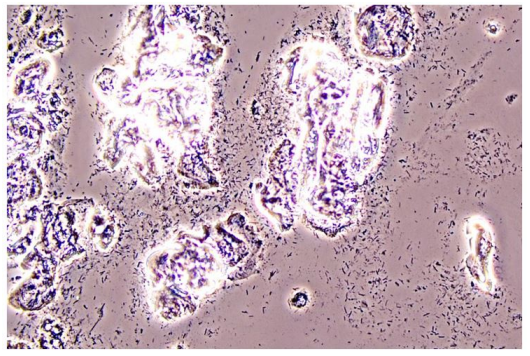
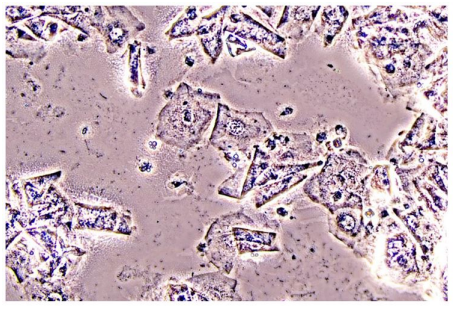
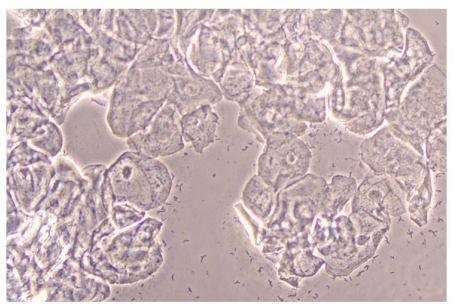
In Figure 1 is a typical microscopical picture of is grad 1 (normal lactobacilli) and Figure 2 is grad 3 (BV) in modified Hay/Ison and Figure 2 is a grad 3 (BV)
Figures 3-8 are some examples for the discrepancies between the Aptima BV Assay, Dynamic Code assay, and the microscopy using the modified Hay/Ison method.
Discussion
The current routine diagnostic methods for BV are subjective and may result in incorrect diagnosis and treatment. However, in recent years, in vitro diagnostic nucleic acid amplification tests (NAATs) have been proposed to be beneficial to improve the accuracy of diagnosing BV. Several studies have shown that these molecular diagnostic methods are superior to the traditional diagnostic methods (Amsel´s criteria and Nugent scores) [4,8]. Indeed, in this study we have shown the potential of molecular diagnostics for BV as the kappa for the studied assays were above 0.80 (after re-examination). The initial microscopic examination was performed by one physician while the reexamination of photographs was performed by two physicians. Surprisingly, in terms of the “test of cure” both molecular methods tested, performed with moderate agreement with modified Hay/Ison kappa index of 0.50-0.60. As the dropout frequency in the second part of the study (“test of cure”) was high, this might have contributed to the low kappa index. Curiously, for most of the samples that resulted in BV with molecular tests, the modified Hay/Ison evaluation showed an intermediate vaginal flora. This rise questions whether the molecular tests are false positive, or the microscopic examination is false negative and are the intermediate vaginal flora a transient or constant state, i.e., will the patient with an intermediate flora after 1-3 months progress into BV or to a normal vaginal flora? To our knowledge, no studies have addressed these questions. However, since the criteria of the intermediate flora vary between different microscopic methods, there is a paramount need for such studies.
The combination treatment of dequalinium chloride vaginal tablets (Fluomizin® or in Nordic countries Donaxyl®), one vaginal tablet for 6 days followed by treatment with 2% clindamycin vaginal cream (Dalacin ®) administered daily for seven days. Had a very high cure rate after 1-3 months (82.8%). During the same time, 499 patients were treated with the same treatment, without being included in the diagnostic test study. Statistics from 200 available tests of cure samples from this patient group showed a cure rate of 82%. The success rate may be explained by starting the treatment with dequalinium chloride vaginal tablets. As this drug is an antiseptic and breaks down the biofilm in the vagina, [20] it will make the bacteria susceptible for the following antibiotic treatment with clindamycin cream.
The microbiome of the vagina has been studied [3]. A normal microbiome in the vagina is dominated by lactobacilli, while an altered, disrupted vaginal flora, vaginal dysbiosis, is dominated by other, often anaerobic, bacteria [3]. BV is just one explanation for vaginal dysbiosis, which can include several other types of vaginal infections. Donders [21] has introduced aerobic vaginitis as another type of vaginal infection.
Today, the diagnosis of BV and VVC is subjective and troublesome, which contributes to the difficulty to perform equivalent clinical studies of different treatment strategies. Thus, several different treatments with low cure rates have been introduced, several of which are sold as over-thethe-counter drugs. With a more accurate diagnosis of BV, results from clinical studies will be more comparable. Still to date, women's vaginal health is not being addressed with the priority and seriousness it should.
Vaginal dysbiosis is a major threat to women’s health [22] and not only a matter of odorous smell. Women with improper vaginal flora have a higher risk of fertility problems, preterm birth, postoperative infections after vaginal surgery [23], and a higher risk of other genital infections, thus an early and correct diagnosis, to be able to provide an adequate treatment, is essential. This study shows that using molecular diagnostic methods are beneficial for this purpose. Molecular methods are objective and work exemplarily on self-collected vaginal swabs. Furthermore, the use of molecular technology will increase the understanding of the underlying mechanisms of the development of vaginal dysbiosis.
Conflicts of Interest to Declare
Per-Göran Larsson has recived a speaker fee from Hologic. He is a scientific advisor for Dynamic code in Linköping, Sweden. Malin Farnebäck and Anna Ansell-Schultz are employed at Dynamic code in Linköping, Sweden.
Funding
Research fund at Skaraborg Hospital, Unicare analyzed the tests provided by Hologic. Hologic has not provided any other funding or support for the study. Dynamic code did analyze the molecular assays free of charge and Per-Göran Larsson has received economic funding from Dynamic code.
Acknowledgement
We thank the youth clinic in Skaraborg for the support of this study.
- Cartwright CP, Lembke BD, Ramachandran K, Body BA, Nye MB, Rivers CA, et al. (2012) Development and validation of a semiquantitative, multitarget PCR assay for diagnosis of bacterial vaginosis. J Clin Microbiol, 50: 2321-9.
- Schwebke JR, Gaydos CA, Nyirjesy P, Paradis S, Kodsi S, Cooper CK (2018) Diagnostic Performance of a Molecular Test versus Clinician Assessment of Vaginitis. J Clin Microbiol, 56.
- Krog MC, Hugerth LW, Fransson E, Bashir Z, Nyboe Andersen A, et al. (2022) The healthy female microbiome across body sites: effect of hormonal contraceptives and the menstrual cycle. Hum Reprod, 37: 1525-43.
- Amsel R, Totten PA, Spiegel CA, Chen K, Eschenbach DA, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med, 74: 14-22.
- Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK (1988) Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol, 158: 819-28.
- Weissenbacher ER, Donders G, Unzeitig V, Martinez de Tejada B, Gerber S, et al. (2012)Fluomizin Study Group. A comparison of dequalinium chloride vaginal tablets (Fluomizin®) and clindamycin vaginal cream in the treatment of bacterial vaginosis: a single-blind, randomized clinical trial of efficacy and safety. Gynecol Obstet Invest, 73: 8-15.
- Gardner HL, Dukes CD (1995) Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified as non-specific vaginitis. Am J Obstet Gynecol. 69: 962- 976.
- Nugent RP, Krohn MA, Hillier SL (1991) Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol, 29: 297-301.
- Larsson PG, Carlsson B, Fahraeus L, Jakobsson T, Forsum U (2004) Diagnosis of bacterial vaginosis: need for validation of microscopic image area used for scoring bacterial morphotypes. Sex Transm Infect, 80: 63-7.
- Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J (1994) Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ, 308: 295-8.
- Ison CA, Hay PE (2002) Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex Transm Infect, 78: 413-5.
- Larsson PG, Breding K, Vikström I, Larsson J (2018) Validation of Hay/Ison Criteria for A Diagnosis of Bacterial Vaginosis Using an Air-Dried Wet Smear. J J Gynec Obst, 5: 038.
- Menard JP, Fenollar F, Raoult D, Boubli L, Bretelle F (2012) Self-collected vaginal swabs for the quantitative real-- time polymerase chain reaction assay of Atopobium vaginae and Gardnerella vaginalis and the diagnosis of bacterial vaginosis. Eur J Clin Microbiol Infect Dis, 31: 513-8.
- Shipitsyna E, Zolotoverkhaya E, Chen CY, Chi KH, Grigoryev A, Savicheva A, et al. (2013) Evaluation of polymerase chain reaction assays for the diagnosis of Trichomonas vaginalis infection in Russia. J Eur Acad Dermatol Venereol, 27: e217-23.
- Schwebke JR, Gaydos CA, Nyirjesy P, Paradis S, Kodsi S, Cooper CK (2018) Diagnostic Performance of a Molecular Test versus Clinician Assessment of Vaginitis. J Clin Microbiol, 56.
- Richter S, Otiso J, Goje O, Voger S, Aebly J, et al. (2020) Prospective evaluation of molecular assays for diagnosis of vaginitis. J clin Micobiol, 58: 01264-19.
- Breding K, Vikström I, Selbing A, Farnebäck M, Hermelin A, Larsson PG (2020) Diagnosis of Bacterial vaginosis using a novel molecular real-time PCR test. J Women’s Health Gyn, 7: 1-7.
- Larsson PG, Forsum U (2005) Bacterial vaginosis-a disturbed bacterial flora and treatment enigma. APMIS. 113: 305-16.
- Larsson PG, Breding K, Vikström I, Larsson J, Farnebäck M, Hermelin A (2020) A Test of Cure Using a New Molecular Diagnostic Approach for Bacterial Vaginosis in Infected Women Treated with Dequalinium Chloride Vaginal Tablets. J Womens Health Gyn, 7: 1-12.
- Gaspar C, Rolo J, Cerca N, Palmeira-de-Oliveira R, Martinez-de-Oliveira J, Palmeira-de- Oliveira A, (2021) Dequalinium Chloride Effectively Disrupts Bacterial Vaginosis (BV) Gardnerella spp. Biofilms. Pathogens, 10: 261.
- Donders GG, Larsson PG, Platz-Christensen JJ, Hallén A, van der Meijden W, Wölner- Hanssen P (2009) Variability in diagnosis of clue cells, lactobacillary grading and white blood cells in vaginal wet smears with conventional bright light and phase contrast microscopy. European Journal Obstetrics Gynecology Reproducitve Biology, 145:109-12.
- Buonarotti U, Debelius JW, Du J, Hugerth LW, Danielsson H, et al. (2022)The vaginal microbiome and the risk of preterm birth: a systematic review and network meta-analysis. Sci Rep, 12: 7926.
- Chen X, Lu Y, Chen T, Li R (2021) The Female Vaginal Microbiome in Health and Bacterial Vaginosis. Front Cell Infect Microbiol, 11: 631972.

Tables at a glance
Figures at a glance