Elsberg Syndrome Due to Genital HSV-2 Reactivation: A Case Report
Received Date: June 20, 2024 Accepted Date: July 20, 2024 Published Date: July 23, 2024
doi: 10.17303/jwhg.2024.11.105
Citation: Liviero S, Driul L, Vizzielli G (2024) Elsberg Syndrome Due to Genital HSV-2 Reactivation: A Case Report. J Womens Health Gyn 11: 1-10
Abstract
Introduction: Herpes simplex virus is a common sexually transmitted infection worldwide. Complications occur in a minority of patients. Acute urinary retention with loss of sacral sensation can occur due to lumbosacral radiculitis secondary to severe HSV infection, featuring cauda equina syndrome with myelitis (Elsberg syndrome).
Case Presentation: A 28-year-old woman presented to our emergency room with a suspected recurrent HSV genital infection and she was treated with Acyclovir orally for 5 days. Two days after finishing the oral therapy, when the local ulcers were already disappeared, neurologic symptoms started. After three days the patient came back to emergency room and was sent for a neurological examination. The symptoms were urinary and fecal retention, paresthesia in vulvar and anal regions, lumbo-sacral pain. After the neurological examination, the patient was immediately hospitalized, underwent a diagnostic LP and a MRI that were consistent with myeloradiculopathy. IV administration of Acyclovir was immediately started and Corticosteroids were added from the 10th day of antiviral therapy.
Discussion: As ES is poorly defined and rarely reported, it can be unrecognized in patients with acute cauda equina syndrome (CES). Appropriate testing for viral infection in a timely manner facilitates reaching a definitive diagnosis and prompt initiation of treatment, which is essential for resolution of symptoms.
Keywords: HSV-2; Elsberg Syndrome; Genital Herpes; Cauda Equina; Acyclovir
Introduction
Genital Herpes Virus Infection
Herpes simplex virus (HSV-1 or HSV-2) is a common infection worldwide.
HSV virus infection is of increasing public health importance. The recurrent nature of the infection, its differing clinical manifestations, and complications such as aseptic meningitis and neonatal infection, are of great concern to patients and health care providers.
Both HSV-1 and HSV-2 can cause genital herpes (but most cases of recurrent genital herpes are caused by HSV-2).
CDC estimated that in the United States, 12 % of people aged 14 to 49 years have HSV-2 infection [1,2].
However, the prevalence of genital herpes infection is higher than that because an increasing number of genital herpes infections are caused by HSV-1.
Genital HSV is frequently under-recognized because infection is often subclinical [3,4]. Its transmission is sexually related.
It is estimated that the majority of genital herpes infections are transmitted by persons unaware that they have the infection or are asymptomatic when transmission occurs (during periods of subclinical viral shedding) [5,6].
The clinical manifestations of genital herpes simplex virus (HSV) vary widely depending upon whether the infection is primary, nonprimary or recurrent.
Primary Infection
The average incubation period for developing genital herpes after an exposure is four days (range 2 to 12 days). The initial presentation can be severe with painful genital ulcers, dysuria, tender local inguinal lymphadenopathy and systemic symptoms such as fever, headache and myalgia. The characteristic skin lesions of HSV infection begin as grouped 2 to 4 mm vesicles with associated underlying erythema that progress to pustules, erosions, and ulcerations [7].
Nonprimary Infection
Nonprimary first episode infection refers to the acquisition of genital HSV-1 in a patient with preexisting antibodies to HSV-2 or the acquisition of genital HSV-2 in a patient with preexisting antibodies to HSV-1. It is associated with fewer lesions and less systemic symptoms than primary infection, presumably because antibodies against one HSV type offer some protection against the other [7].
Recurrent Infection
Most cases of recurrent genital herpes are caused by HSV-2. Clinical recurrences of genital HSV are common but are typically less severe than primary or nonprimary infections. The mean duration of lesions is generally shorter in recurrences than in primary infection (10 versus 19 days) and the duration of viral shedding is usually two to five days.
Atypical vaginal lesions include fissures or vulvar irritation. Systemic symptoms are infrequent and approximately 25% of recurrent episodes are completely asymptomatic. 50% of patients with symptomatic recurrences have prodromal symptoms before eruption such as local mild tingling or shooting pains in the buttocks, legs, and hips [8,9].
Extragenital manifestations (e.g., radiculitis, aseptic meningitis, urinary retention) typically occur during the primary episode of herpes simplex virus (HSV) infection, but they can recur with subsequent episodes.
Neurological complications occur in a minority of patients who present with primary herpes simplex virus (HSV) infection. One study reported aseptic meningitis in 8 % of cases and urinary bladder retention due to sacral autonomic nervous system dysfunction in 2% of cases. [8] Other series have noted higher rates of aseptic meningitis (25 %) and urinary retention syndromes (10 to 15 %) in women with primary infection [10].
In HSV meningitis the cerebrospinal fluid (CSF) profile includes a pleocytosis (median white cell count 300 to 400/mm3) with a predominance of lymphocytes, and a normal CSF glucose concentration [8].
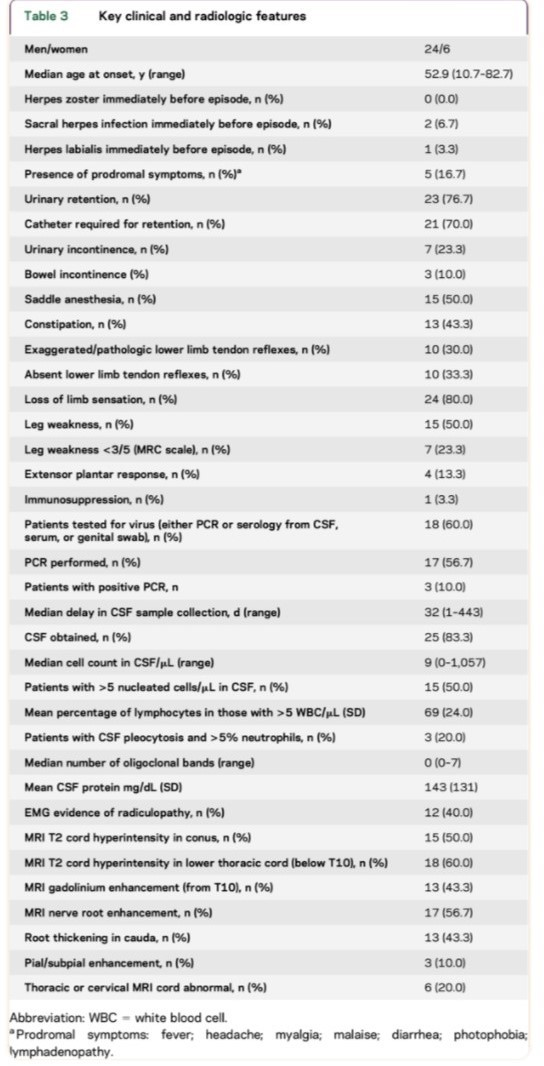
Lumbosacral radiculitis can occur generally secondary to severe primary HSV infection. It can lead to acute urinary retention with loss of sacral sensation. In a series of 30 patients who developed acute lumbosacral radiculitis featuring cauda equina syndrome with myelitis (Elsberg syndrome), urinary retention was present in 77 %, and 50% had lower extremity weakness [11,12].
The clinician should differentiate dysuria from acute urinary retention. Dysuria can lead to reluctance to void because of the passage of acidic urine on open and inflamed vesicles, whereas acute urinary retention with loss of sacral sensation can occur due to lumbosacral myeloradiculopathy secondary to severe primary HSV infection.
This complication is transient but usually requires catheterization until clinical improvement ensues.
Elsberg Syndrome
In 1914, neurosurgeon C. A. Elsberg and colleagues reported the similar cases of five young adults with a syndrome of urinary retention, constipation, and radicular pain, paraesthesia, and paresis of the lower limbs. The aetiology was unknown but presumed infectious or toxic [13].
Neurotropic viruses, primarily herpes simplex virus type 2 (HSV-2), were later identified as a cause of lumbosacral radiculitis or myeloradiculitis, and hence the term Elsberg syndrome has been used for this condition [14].
According to the conclusions of different authors, a more insidious onset of the disease suggests non-infectious aetiology. The aetiology remains unidentified in a proportion of patients [12,15].
Elsberg syndrome (ES) is a self-limiting neuroinflammatory disease that causes acute or subacute lumbosacral radiculitis, with or without myelitis, with CSF pleocytosis (Table 1). It is in most cases a manifestation of reactivation, or occasionally, primary HSV-2 infection. Other infectious agents are SARS-CoV-2, West Nile Virus, Varicella Zoster Virus (VZV), Epstein–Barr virus, Cytomegalovirus, HIV, and HSV-1.
ES typically presents as cauda equina syndrome (CES), with symptoms of sensory impairment, lower extremity weakness, saddle anesthesia, and urinary and/or bowel retention. It accounts for 5-10% of CES. [12, 16, 17]
Thus, ES has to be considered in the differential diagnosis of acute cauda equina syndrome (CES) and performing appropriate testing for HSV infection can facilitate prompt diagnosis and treatment.
As previous research has been limited to case reports and a single retrospective cohort studies, further systematic description is required to improve timely diagnosis and treatment. [14, 15, 18 – 22, 23, 24, 25, 28]
Case Presentation
We report the case of an immunocompetent 28- year-old woman who presented with perineal paresthesia, urinary retention and constipation associated with lumbo-sacral pain. A week before, an episode of recurrent genital HSV-2 infection manifestation preceded the clinical presentation, and it was treated with Acyclovir 400mg orally 5 times a day for 5 days.
Immediately after an urgent neurologic evaluation, the patient was hospitalized with the diagnosis of cauda equina syndrome secondary to HSV infection.
Lumbar puncture was performed immediately after the admission, in order to check for cell, count determination and neurotropic viruses and to start subsequently intravenous antiviral therapy.
The cerebrospinal fluid (CSF) white blood cell count was 135 leukocytes/μL, with a lymphocyte predominance, and glucose levels were normal, as we expect in a viral infection. Thus, the cell count determination supported the diagnostic suspicion of a viral infection (Table 2).
The CSF multiplex molecular detection for viruses was negative. Despite this, intravenous antiviral therapy was started, given the strong clinical suspicion of HSV-2 reactivation.
The second day of hospitalization, a magnetic resonance imaging (MRI) with contrast was performed. It showed T11-T12 spinal cord hyperintensity and first nerve roots of dural sac hyperintensity. Sacral nerve roots didn’t appear involved. The hyperintensity is a sign of involvement of the mentioned structures.
The injection of gadolinium showed L1 enhancement, conus appearing not involved. These radiological findings were consistent with myeloradiculopathy, since the hyperintensity and later the enhancement after gadolinium injection are direct signs of involvement.
The Pap (Papanicolau) Test performed as regular screening 10 days before hospitalization resulted in viral cytopathic alterations in the cervix. A specific analysis was required on that cytological swab by the neurologists, in order to know the specific virus: HSV-1 HSV-2 or VZV. After two months, the results showed a HSV type 2 infection, confirming the hypothesis.
Regarding the therapy, intravenous administration of Acyclovir was immediately started and continued for 12 days, then Corticosteroids were added from the 10th day of antiviral therapy, after the evaluation of an infectious disease specialist (IV administration of Dexamethasone).
The day after the LP, due to the onset of nuchal rigidity, headache and spinal pain and consequent psychomotor agitation, the patient was administered Diazepam IV to relieve the symptoms.
At the hospital discharge, the patient was prescribed an outpatient oral therapy with Valacyclovir 1000mg twice a day for 7 days (for a total duration of 18 days of antiviral therapy) and Dexamethasone orally for 10 days, gradually decreasing the dose.
As regards the symptomatology, the symptoms gradually improved, with a return to spontaneous urination after 2 days (without the need of catheterization) and defecation after 5 days of therapy. In the meanwhile, evacuating enemas were administered. Paresthesias disappeared in a month. As regards the lumbosacral pain, it gradually improved but persisted for months. The neuropathic pain was treated with Pregabalin 100 mg daily for two months and physiotherapy rehabilitation for three months.
Discussion
As ES is poorly defined and rarely reported, it can be unrecognized in patients with acute cauda equina syndrome (CES). Appropriate testing for viral infection in a timely manner facilitates reaching a definitive diagnosis and prompt initiation of treatment, which is essential for resolution of symptoms. Clinical, radiological and laboratory findings can help early diagnosis.
Autonomic nervous system dysfunction leads to several symptoms that can present in a variety of associations: sensory impairment like numbness and tingling of the perineal area, saddle anesthesia, urinary retention and constipation, lower extremity weakness. Rarely, transverse myelitis associated with the development of a rapidly progressive symmetrical paralysis of the lower extremities has been reported.
There are no pathognomonic or specific radiologic findings. Nerve root enhancement often occurs contemporaneously with spinal cord involvement, although either nerve root enhancement or spinal cord parenchymal signal abnormality can predominate; the cord lesions in ES are not consistently located in the most caudal portion of the conus.
Cerebrospinal fluid (CSF) examination often demonstrates lymphocytic pleocytosis and normal glucose levels, as it is expected in a viral infection.
But HSV-2 DNA is not always detected in the CSF, as reported by Savoldi et al. [12] First of all, the diagnostic accuracy of CSF viral detection is imperfect in real-life settings, therefore a negative result cannot exclude the presence of infection [26]. Furthermore, a rapid viral clearance from CSF has been shown for HSV [27].
Lastly, the absence of HSV-2 DNA detection in some of Elsberg syndrome CSF samples might be explained by an immuno-mediated pathophysiological process underlying radiculomyelitis [28].
The pathogenesis of autonomic nervous system dysfunction is not completely understood.
HSV-2 is the most common pathogen, the aetiology remained unidentified in a substantial proportion of patients [15].
HSV-2 is a well-known pathogen of the central nervous system and is dormant in 40% of sacral dorsal root ganglia; when reactivated, virus can spread axonally into the spinal cord leading to various manifestations. The same mechanism happens in a primary infection [11,25].
As Nsoga et al reported, the absence of HSV-2 DNA detection in some of CSF samples might be explained by an immuno-mediated pathophysiological process underlying radiculomyelitis [28].
Craig et al hypothesized that hematogenous spread to the central nervous system or meninges accounted for its pathogenesis [29].
In animal models central nervous system involvement appears to result from direct spread of HSV from mucosal sites via peripheral nerves [30]. Neurotropic spread in animals is often associated with clinical paralysis or death due to encephalitis, manifestations of disease that have been rarely reported in aseptic meningitis associated with genital herpes in humans. The pathogenesis of HSV meningitis is unclear. Further research is necessary to better understand whether hematogenous, neurotropic, or a combination of both these methods are means by which the virus reaches the central nervous system.
As regards the treatment, it is not validated due to the rarity of this illness. There are insufficient case reports to draw conclusions on the best approach or optimal duration of treatment.
The antiviral-corticosteroid combination is based on the idea that both direct viral and inflammatory mechanisms could be implicated in the pathogenesis.
The administration of Acyclovir is appropriate even in the absence of demonstration of viral infection given the favorable risk-benefit profile of this drug. HSV and VZV are treatable when Acyclovir is initiated early, but the potential benefits of treatment with antiviral drugs have not been documented. Antiviral treatment may affect symptoms’ duration, but there is no evidence it helps with neurologic improvement in herpetic myeloradiculopathy.
Since inflammatory mechanisms could be implicated in the pathogenesis, steroids can be initiated to reduce inflammation, but also the use of corticosteroids in the treatment of ES is debated. They can be used to help shorten the duration of symptoms [12,23,25,28].
In conclusion, being conscious of Elsberg syndrome while treating patients with signs and symptoms of lumbosacral radiculitis is crucial as initiating IV Acyclovir along with Corticosteroids is regarded as a treatment of choice due to the possibility of shortening symptoms duration and improving overall morbidity.
This case presentation, in addition to the series of cases present in literature [15,23,24,25,28], has the aim to help other clinicians in the early diagnosis and subsequent treatment of this rare complication of genital herpes infection.
Acknowledgment
Published with written consent of the patient.
Conflict of Interest
None declared.
Data Availability
The data are available, but restrictions apply to the availability of these data, which was used under license for the current article, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Internal Review Board.
Author Contributions
All authors read and approved the final version of the manuscript.
S.L.: contributed to the evaluation and management of the patient, collected information and was the writer of the manuscript.
G.V.: reviewed the manuscript.
L.D.: contributed to the evaluation and management of the patient and reviewed the manuscript.
- Bernstein DI, Bellamy AR, Hook EW 3rd, et al. (2013) Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 56: 344.
- McQuillan G, Kruszon-Moran D, Flagg EW, Paulose-Ram R (2018) Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14–49: United States, 2015–2016. NCHS Data Brief, no 304. Hyattsville, MD: National Center for Health Statistics.
- Benedetti J, Corey L, Ashley R (1994) Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 121: 847.
- Schillinger JA, McKinney CM, Garg R, et al. (2004) Seroprevalence of herpes simplex virus type 2 and characteristics associated with undiagnosed infection: New York City, 2004. Sex Transm Dis. 35: 599.
- Xu F, Sternberg MR, Kottiri BJ, et al. (2006) Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA, 296: 964-73.
- Workowski KA, Bachmann LH, Chan PA, et al. (2021) Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 70:1.
- Kimberlin DW, Rouse DJ (2004) Clinical practice. Genital herpes. N Engl J Med. 350: 1970.
- Corey L, Adams HG, Brown ZA, Holmes KK (1983) Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 98: 958.
- Wald A, Zeh J, Selke S, et al. (1995) Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 333: 770.
- Whitley RJ, Kimberlin DW, Roizman B (1998) Herpes simplex viruses. Clin Infect Dis. 26: 541.
- Eberhardt O, Küker W, Dichgans J, Weller M (2004) HSV-2 sacral radiculitis (Elsberg syndrome). Neurology. 63: 758.
- Savoldi F, Kaufmann TJ, Flanagan EP, et al. (2017) Elsberg syndrome: A rarely recognized cause of cauda equina syndrome and lower thoracic myelitis. Neurol Neuro immunol Neuro inflamm, 4: e355.
- Kennedy F, Elsberg CA, Lambert C (1914) A peculiar undescribed disease of the nerves of the cauda equina. Am J Med Sci. 147: 645-67.
- Hemrika DJ, Schutte MF, Bleker OP (1986) Elsberg syndrome: a neurologic basis for acute urinary retention in patients with genital herpes. Obstet Gynecol. 68: 37S-9.
- Petersen PT, Bodilsen J, Jepsen MPG, et al. (2024) Danish Study Group of Infections of the Brain (DASGIB). Viral lumbosacral radiculitis (Elsberg syndrome) in Denmark. Infection. 52: 839-46.
- Oates JK, Greenhouse PR (1978) Retention of urine in anogenital herpetic infection. Lancet. 1: 691-2.
- Whalen AM, Mateo CM, Growdon AS, Miller AF (2019) Sacral myeloradiculitis: an uncommon complication of genital herpes infection. Pediatrics. 144: e20182631.
- Caplan LR, Kleeman FJ, Berg S (1977) Urinary retention probably secondary to herpes genitalis. N Engl J Med. 297: 920-1.
- Nakajima H, Furutama D, Kimura F, et al. (1998) Herpes simplex virus myelitis: clinical manifestations and diagnosis by the polymerase chain reaction method. Eur Neurol. 39: 163-7.
- Suarez-Calvet M, Rojas-Garcia R, Querol L, Sarmiento LM, Domingo P (2010) Polyradiculoneuropathy associated to human herpesvirus 2 in an HIV-1-infected patient (Elsberg syndrome): case report and literature review. Sex Transm Dis. 37: 123-5.
- Aurelius E, Forsgren M, Gille E, Skoldenberg B (2002) Neurologic morbidity after herpes simplex virus type 2 meningitis: a retrospective study of 40 patients. Scand J Infect Dis. 34: 278-83.
- Yoritaka A, Ohta K, Kishida S (2005) Herpetic lumbosacral radiculoneuropathy in patients with human immunodeficiency virus infection. Eur Neurol. 53: 179-81.
- Abdullah AAN, Tallantyre E (2019) HSV-2 radiculitis: An unusual presentation mere days after genital infection. Clin Neurol Neurosurg. 185: 105429.
- Nishiyama D, Yoshimura S, Shimizuhira C, et al. (2023) Elsberg syndrome caused by herpes zoster in the sacral region with preceding urinary retention. Acute Med Surg. 10.
- Belfaqeeh O, Markley A, Patel M, et al. (2023) Elsberg syndrome in HSV-2 infection. ID Cases. 31.
- Davies NW, Brown LJ, Gonde J, et al. (2005) Factors influencing PCR detection of viruses in cerebrospinal fluid of patients with suspected CNS infections. J Neurol Neurosurg Psychiatry. 76: 82-7.
- Kleines M, Scheithauer S, Schiefer J, et al. (2014) Clinical application of viral cerebrospinal fluid PCR testing for diagnosis of central nervous system disorders: a retrospective 11-year experience. Diagn Microbiol Infect Dis. 80: 207-15.
- Nsoga MTN, Accorroni A, Mamin A, et al. (2022) Primary HSV-2 Infection Complicated by Radiculomyelitis in a Young Immunocompetent Female Patient with Inherited Chromosomally Integrated HHV-6: A Case Report. Viruses. 14: 1979.
- Craig C, Nahmias A (1973) Different patterns of neurologic involvement with Herpes simplex virus types 1 and 2: isolation of Herpes simplex virus from the buffy coat of two adults with meningitis. / Infect Dis. 127: 365-72.
- Overall JC Jr, Kern ER, Schlitzer RL, Friedman SB, Glasgow LA (1975) Genital herpesvirus hominis infection in mice. I. Development of an experimental model. Infect Immun. 11: 476-80.

Tables at a glance